INTRODUCTION
Genetically Modified Organism is an alternative way to improve both the quality and the quantity of agricultural products. In developing nations across the globe, governments are grappling with questions of what role, if any, genetically modified organisms should play in helping address a range of themes and issues: agriculture; nutrition; and climate challenges. (Cornis, 2018). The outputs of policy making in Indonesia is to ensure consumer protection by food labeling of GMO products. Indonesia has significant capacity to promulgate but limited capability to enforce regulations with respect to biosafety of GE products (USDA Foreign Agricultural Service, 2015). Indonesia’s regulations on GMO itself is still dependent on worldwide statement. This study aims to provide information on how Indonesian regulations address issues of GMO and how controversies occur within the Indonesian social sphere towards GM food consumption. The controversies and issues that will be discussed are around the environment, safety of consumption, biosafety, health impacts, cultural and religious issues.
Genetically Modified Food Defined
Genetically Modified Food (GMF) products are foods produced or that use raw materials, food additives, and/or other materials produced, from the genetic engineering process (National Agency of Drug and Food Control, 2018). Genetic engineering is defined as a biotechnology technique that is carried out by moving genes from one living creature to another. The transfer of the gene is referred to as GMO. Gene transfer can occur in the same or different species, for example from microorganism genes to plants or animals. The purpose of genetic engineering is to produce living things in the form of plants, animals, or microorganisms/microorganisms that have certain properties which are beneficial to humans. (Sumarto, 2017). The benefits of Genetic Engineering or GMO products are to improve the quality of plants so that plants are resistant to pests and diseases, resistant to drought stress, high salt content, frost resistant, and help improve the quality of nutrient content.
GMF in Indonesia
GMF is one of the results of a breakthrough in the application of biotechnology to increase food production. In Indonesia, many of these products have been mainly imported fruits and meat, as well as imported vegetables and imported food products. Most products containing GMO are vegetable and fruit products such as apples, oranges, bananas, and many more.
Indonesia still has not succeeded in developing GMO plants. All over the world, the development of genetically engineered food has been more advanced and is increasingly widespread. The United States is one country that has used transgenic seeds such as corn, tomatoes, potatoes, and papaya. (Anindyaputri, 2017).
The social problems and challenges faced in the circulation of GMF products, especially in Indonesia, are plants that have to do limited field testing and require a long time so that the process of implementing GMF products becomes long. Government regulations that are still unclear can trigger public confusion about the safety of consuming GM food products. Further research studies on GM products are still continuing but do not provide significant results, especially directly to the community. However, GM products have been circulating and most of the Indonesian population have consumed them. This became viral and controversial because many studies did not agree with GMO products. Various considerations such as the national economy, national food self-sufficiency and the government's mission to stabilize food prices nationally are still supporting factors for GMO products in Indonesia.
Significant increase in Indonesia’s population
The population in Indonesia has increased rapidly since a few years ago (refer to Fig. 1). Based on the projection of the National Development Planning Agency, the Central Statistics Agency, and the United Population Fund, Indonesia's population will reach 271 million by 2020. (Central Bureau of Statistics, 2018a). This has resulted in increased consumer demand for staple foods, while domestic food production is unable to support consumer demand.
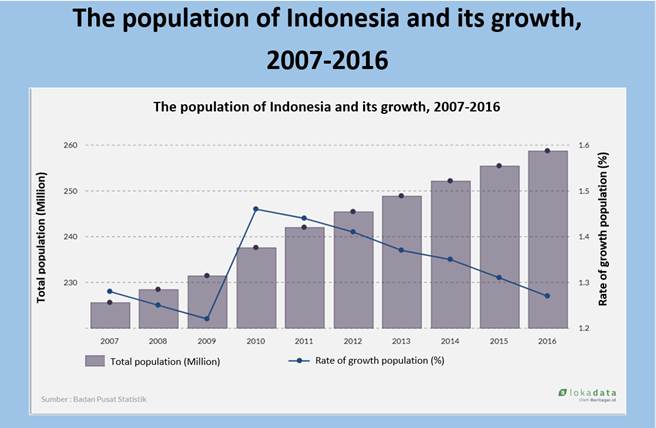
Fig. 1. The Growth of Indonesia's population from 2007 to 2016 (Central Bureau of Statistics, 2017)
Instability of national food production
Domestically, many food productions are hampered by very erratic climate such as droughts or floods that eventually reduce food production in Indonesia. According to the Association of Indonesian Seed and Farm Technology Banks, in 2018, the current drought has the potential to reduce production potential by up to 60%. Unstable rice production, from January to March 2017, reaching 15.6 million tons. The details, production in January amounted to 2.8 million tons, February 5.4 million tons, and March 7.4 million tons. (Warta Ekonomi, 2018). As a result, Indonesia relies on imports of agricultural products to meet national needs. This also has a direct impact on the instability of rice prices every month.
Based on data from the Central Bureau of Rice Import Statistics up to the first semester of 2018, it has reached 1.12 million tons, which means it has jumped 755% compared to the first semester of 2017. Similarly, the value of rice imports in the first six months of this year surged more than 1600% to US$ 524.3 million.
During the second quarter of this year, rice imports reached 736,000 tons, up to 91.84% from the previous quarter and also jumped 765% compared to the same quarter last year. (Central Bureau of Statistics, 2018b).
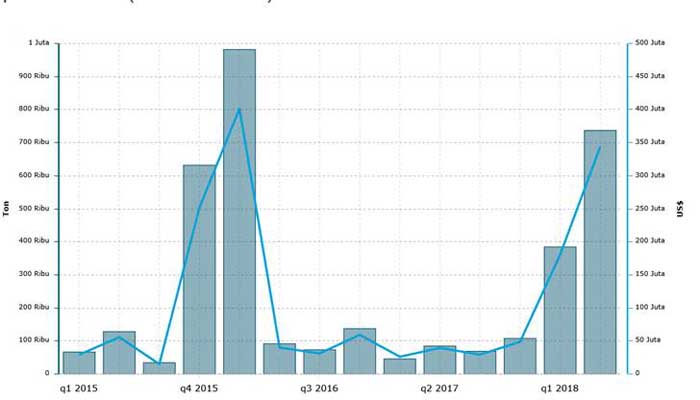
Fig. 2. Indonesia’s rice import history 2015-2018. (Central Bureau of Statistics, 2018b)
Legal aspects of GMF in Indonesia
- Law of The Republic of Indonesia, Act No 7 the of 1996 about Food
- The act of The Republic of Indonesia No 21 the Year of 2004 about Ratification of Cartagena Protocol on Biosafety
- Government Regulations of the Republic of Indonesia No 69 the of 1999 about Food Labelling and Advertisement
- Government Regulations of the Republic of Indonesia No 28 the of 2004 about Food Safety, Quality, and Nutrition
- Government Regulation of the Republic of Indonesia No 21 the of 2005 about Biosafety of Genetically Modified Organism
- Presidential Regulation of the Republic of Indonesia No 39 the of 2010 about Biosafety Commission of Genetically Modified Organism
- Head of NADFC Regulation Number: HK.00.05.23.3541 Year of 2008 about Guidelines for Food Safety Assessment of Genetically Modified Organism (Sparringa, 2010)
- Regulation of Drug and Food Supervisory Agency (BPOM) No 6 the of 2018 about The Supervision of Food Genetic Engineering Products
Indonesia’s regulation on GMF
Genetic modifying technology has been developed in Indonesia since the 1990s and as a result of new technology, is necessary to manage the product settings to prevent some causes like bad influences on humans, animals and the environment, especially its effect on overall biodiversity. The regulatory and management of Indonesia’s biological safety have been established by Governmental Regulation No. 21 of 2005 about GMO Biosafety and Presidential Regulation No. 39 of 2010 about Commission of Biosafety of GMO, which provide recommendations to the ministries and involved agencies related in the prerelease of GMO. (Deswina, et al, 2013).
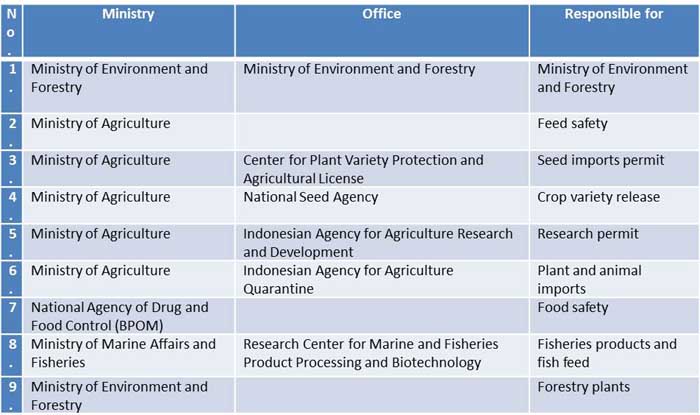
The national competent authority for GM products
Table 1. The national competent authority list for GM Products (USDA Foreign Agricultural Service, 2015) (as cited in Indonesia Biosafety Clearing House, 2010; FAS, 2012)
GMF product distribution regulation in Indonesia
The Biosafety Commission is responsible for giving recommendations to the Minister and Head of Non-Ministry Government Institutions, authorized to prepare and determine policies and issue PRG biosafety certificates.
In the food business chain, anyone who produces or imports GMF products such retailers and distributors for food trading purpose must fulfill food safety, quality, and nutritional requirements in accordance with the legislation standards. In food business those who produce GM food domestically and/or import GM food for trading in retail packaging must include labels in accordance with the provisions of the legislation.
Points of consideration in Genetic Modified Food in Indonesia: (Indonesia Biosafety Clearing House, 2012)
- Genetic Modified Food is synthetic, so it has a risk of instability, allowing unexpected transfer of genes, which can lead to unexpected traits. For reasons of threats to conservation, it is necessary to have proper regulations, regulated by Law of Republic of Indonesia No. 21 the Year of 2004 and Governmental Regulation No. 21 the Year of 2005 concerning Biosafety of Genetically Engineered Products.
- Biosafety is a condition that prevents the possibility of risks that are detrimental to animal and human biodiversity health as a result of the use of genetically engineered products.
Indonesia has regulated the circulation of GMF products in the domestic market. Before being distributed for public consumption, GM product foods must be reviewed and examined. This policy started since 1996 when the enactment of Law No. 7 the Year of 1996 concerning Food currently being revised into Law No. 18 the Year of 2012 concerning Food. In addition, Indonesia has ratified Cartagena Protocol on Bio-Safety to the Convention on Biological Diversity to become Law No. 21 the Year of 2004 concerning the Ratification of Cartagena Protocol on Bio-Safety to The Convention on Biological Diversity which applies the precautionary approach in the handling of GMOs. (National Agency of Drug and Food Control, 2017).
To implement this policy, the Government has compiled legislation related to the security assessment of GMF products, namely:
- Republic of Indonesia Government Regulation Number 69 the Year of 1999 concerning Labels and Food Ads
- Government Regulation No. 28 the Year of 2004 concerning Food Safety, Quality, Nutrition;
The Indonesian Ulama Council has issued an Islamic Law (fatwa) regarding PRG food through the Fatwa of the Indonesian Ulama Council Number 35 the Year of 2013 concerning Genetic Engineering Organism and its products.
International policy background
The United States supports the use of GMOs as part of the strategy to achieve its agricultural development goals. In 2002, USAID launched the Collaborative Agricultural Biotechnology Initiative (CABIO) “to promote developing country access to and management of new scientific tools such as biotechnology for improving agriculture productivity, environmental sustainability and nutrition” (U.S. Department of State and USAID 2005: 191). American will continue to fund biotechnology research and policy activities. This will include a shift from biotechnology research to field trials in Africa and Asia. (Sourcewatch, 2012)
Meanwhile, the European Union’s, Principle 15 of the 1992 Rio Declaration, EU doubts around GMOs stem from a broader interpretation of the precautionary principle, in which GMOs are viewed as foundationally different from previous techniques of selective and mutagenic breeding. Within this act the assessment of non-safety concerns is mandatory, and “can include any health and environmental consequences in the countries in which the crops are grown, notably developing countries. (Schnurr and Smyth, 2016)
In the Association of Southeast Asian Nations ASEAN countries, many still do not have the capacity to develop biosafety clearing-house mechanisms. Moreover, the policy on the national biosafety legal framework is vitally important and should be set as the first priority and be followed by a clear-cut plan of implementation for all the countries in the region. The ASEAN Ministers of Agriculture and Forestry approved the proposal of collaboration with the International Life Sciences Institute (ILSI) to conduct Training Workshop on Safety and Risk Assessment of Agriculture-Related GMOs annually.
Singapore is the only country to step out when describing the situation of agro-biotechnology in Southeast Asia. Singapore’s open import agriculture-related GM organisms is required to submit an application under specified Singaporean standard, which forwards it to the Subcommittee on the Release of Agriculture-Related GMOs. (Adduci, 2014).
Controversy and social issues of GMF in Indonesia
Until now, the controversy over genetically engineered food is still continuing, while GM products continue to be spread throughout almost the entire world and consumed by so many animals and humans.
- For the pro: genetic engineering is the right solution, cheaper prices, better quality, and also removes allergens from food.
- For the contra: the desire for consumer health, the need to create new allergens, replace the balance of native nutrition, the in terms of halal food products, and antibiotic and pesticide resistance.
Controversy began when according to the teaching staff at the Department of Food Science and Technology, Faculty of Agricultural Technology IPB, the negative law about GMF was incorrect, because every GMF variety before being released to the market must go through the testing section, namely: from structural tests, toxicity, testing to animals, released to a limited group, then marketed. When a variation has been released to the community, the Food Safety Commission of the Ministry of Agriculture conducts research and testing in the field.
What is meant by the Alliance Coordinator for Prosperous Villages, the Government of Indonesia regarding GMF products from abroad is safe, because foreign parties have conducted studies using resources and products that are in accordance with PRG that have the same substance, are not feasible towards varieties. The different statements are suggested to be addressed by the government by conducting research on GMF products, and publishing the results so that this anxiety and controversy are not prolonged.
Controversy over genetic engineering products consumed by communities
The existing domestic food products have not been able to overcome the problem of food shortages, and this has become a challenge for agricultural development in Indonesia. Many products that have been marketed are GMO products, but unfortunately, all GMO products in the market are not given clear information, therefore the most prominent problem still causing controversy in the community is if it is safe for GMO products to control the current market for consumption?
Pro GMO groups argue that there is unlimited potential in genetic engineering that is useful for reducing pesticide use, overcoming food shortages, and producing more nutritious foods and medicines. Groups that are against or refuse to think that food products and GMO drugs are not believed to be safe for consumption because they still cause various negative impacts on health and the environment. Another negative impact for farmers, in particular, is very detrimental to them because non-GMO farmers are unable to increase productivity and become more profitable.
The following describes the community controversy regarding the acceptance and use of GMO products in the fields of agriculture, environment, health, religion, culture, and ethics.
GMO controversy in agriculture and the environment
Positive:
In the long term this GMO plant will change the structure and texture of the soil which will have an impact on the quantity and quality of crop production. The positive impact of plants that are capable of producing substances that can eradicate weeds is to reduce costs because they do not need to buy herbicides which are relatively expensive for farmers.
Negative:
Genetically engineered plants have the potential to damage the balance of the surrounding environment. Pests and plant diseases will run into conventional fields so that inevitably, these farmers have to switch to using GMO crops which are relatively expensive. Environmentalists worry that GMO crops will pose environmental risks when they are widely cultivated. Another problem that is expected to emerge is the killing of other living creatures such as butterfly larvae, which in turn are feared because extinction of butterflies as a result of GMO residues is toxic.
GMO controversy in the health sector
Positive:
The production of GMO drugs such as insulin, monoclonal antibodies, anti-allergies, anti-cancer drugs and many other drugs to cure disease sharing has been felt by the community as reported by Singh et al. (2006). The ability to express foreign genes using genetic engineering technology has opened up the option to produce a large number of commercial food products and medicines/pharmaceuticals essential for improving public health.
Negative:
Genetically engineered food is thought to be the cause of various diseases with the assumption that foreign genes might alter the nutritional value of food in unexpected ways that can reduce or increase some other nutrients and nutrients. Factors that need to be considered from the minimum information is that one must be careful in using GMOfood products. Nordlee et al. (1996) reported that Brazil nuts as one of the GMO products were withdrawn from circulation because they caused allergies to consumers. These allergic reactions are thought to be caused by the modification of certain genes.
GMO controversy in the fields of religion, culture, and ethics
Muslim community groups in Indonesia as the majority group have provisions that require the food consumed is halal and good (halal toyyiban), so it is very important to include information about the content of food products and medicines produced by GMOs although it is not easy to trace the contents. The GMO requires a clear mechanism for tracking and monitoring GMO content that is widely circulated. Also, very important aspect is the inclusion of halal certificates issued by the Institute for the Study and Supervision of Medicines and Food of the Indonesian Ulema Council (LP POM MUI) so that the concern of the Muslim community in consuming GMO products is not developing and unsettling.
Food Safety Based on Legal Basis from National Agency of Drug and Food Control
Genetically Modified Food product requirements
- GMO food both from within and outside the country that will be reviewed or tested for distribution in Indonesia must be accompanied by basic information as a guide so that the product meets food requirements and environmental security.
- Basic information as a guideline to meet the food safety requirements:
- Genetic engineering methods are used to follow standard procedures that scientifically account for their validity;
- The substantial nutritional content of GMF must be commensurate with non-GMF ones
- The content of toxic, antigenic and allergic compounds in the GMF must be substantially commensurate with non-GMO ones;
- The carbohydrate, protein, ash, fat, fiber, amino acids, fatty acids, minerals and vitamins in the GMF must substantially be commensurate with those of non-GMOs;
Proteins that are encoded in the transferred gene are not allergens;
The method of extermination is used if there is a deviation.
Indonesia’s nationalstandard food safety information
Substantial value
Determination of substantial equivalence of genetically modified food requires consideration of the characteristics of foodstuffs or processed products which include a of food composition, phenotype and metabolite properties, food processing factors with conventionally obtained food.
Food component composition
Analysis of the composition of the components of PRG food or its processed products carried out for macro nutrients; proximate analysis (crude fiber, carbohydrate ash, fat, protein), fatty acids, amino acids and micronutrients (minerals, vitamins) and analysis of other components found in food that are deemed necessary
Phenotype properties
- GM food from plants includes: shape, size, color, texture
- GM food from animals includes: shape, size, color, aroma, taste
- GM food from fish includes: shape, size, color, aroma, taste and
- GM food from microorganisms species characteristics (aroma morphology, taste and other characteristics in normal conditions and other characteristics of other physiological characteristics, ribotyping) potential colonization, infectivity, plasmid host diversity, antibiotic resistance patterns, and toxicity.
Metabolites
This food safety assessment requires an investigation of residual levels and metabolites in food and an assessment of altered nutrient profiles. If changes are identified in residual or metabolite levels, it must consider the potential impact on human health using conventional procedures to determine the safety of these metabolites or residues.
Food Processing
The potential influence of the food processing process on PRG food must also be taken into account. For example, there can be changes in endogenous toxicity and bioavailability of nutrients.
Changes in nutritional value
GM food products that are intentionally increased in nutritional value (for example with the presence of pro-vitamin A in rice and mustard), an assessment of the nutritional value of the GMF product must be carried out. Information about the consumption patterns of food and its processed products must use a similar approach.
Assessment of the nutritional value of GMF products. Information on the consumption patterns of processed foods must use a substantial similarity approach to conventional food consumption. The potential for undesirable effects can be detected using the approach to food use at the highest consumption level. Requirements for physiological characteristics and metabolites in special population groups such as infants, children, pregnant and lactating women, the elderly and those suffering from chronic diseases or those with damaged immune system needs attention.
Allergenicity
Food allergen city is a side-effect reaction that involves the immune system, namely increasing immunoglobulin E (IgE) in individuals who are very sensitive to special substances contained in food or food components, most food allergens are proteins but can also be hapten (small molecules that are antigen and cause allergies). Assessment of allergenic potential was carried out to assess the allergenicity of GMF products.
Toxicity
Information on PRG food toxicity testing covers at least acute toxicity to new proteins and sub-chronic toxicity to food. In vitro nucleic acid techniques allow the insertion of DNA that can produce a synthesis of new substances in plants. These new substances can be food components, such as protein, fat, carbohydrates and new vitamins in GMOs. The new substance can also be a new metabolite resulting from enzyme activity and the expression of inserted DNA.
Indonesia is still in serious hunger and poverty rates
In the 2018 Global Hunger Index, Indonesia ranks 73rd out of 119 qualifying countries. With a score of 21.9, Indonesia suffers from a level of hunger that is serious. (Global Hunger Index, 2018). More than 19 million Indonesians are still facing malnutrition. The data shown 2 to 3 children for every 100 children die before they are 5 years old. (Naelufar, 2017)
The Indonesian government claimed to overcome the problem of food sufficiency by increasing supply through increasing rice production and developing higher value crops. However, this strategy is proved to be ineffective because even though Indonesia experienced good economic growth, there were still 19.4 million citizens who could not meet their daily food needs. (Kompas, 2018).
GMO able to reduce dependence on imports and increase domestic food production
The use of biotechnology in agriculture can increase the productivity of food crops so that Indonesia can reduce imports (Yuniar, 2018). Indonesia has researched several genetically engineered plants that can increase farmer productivity because it has pest and disease resistance, one of which is GMF sugar cane event NXI-4T which is the world's first biotech sugar cane. Not only does it have pest and disease resistance, but also GMF is able to improve the quality of food products in Indonesia. Thus, it is not only possible to reduce food imports and also is able to reduce hunger rates in Indonesia.
According to the Professor Emeritus of Economics, Bogor Agricultural University (IPB), Prof. Dr. Parulian Hutagaol, revealed, genetic engineering techniques on food were first developed to answer various problems such as food security and climate change. (Saudale, 2018). Unfortunately, Indonesia still has concerns about biotech crops that disrupt the environment. As a result, environmental, food and feed tests at the government level take a short time.
The holiness of GMF based on Islamic law in Indonesia
A ruling on a point of Islamic law of the Indonesian Ulama Council (MUI) No 35 the Year of 2013 states that genetic engineering products are permitted (mubah) and under conditions of the nature of change genetic engineering products are useful for good reason, are harmless to environmental, do not apply human body genes or human parts. Based on the Islamic law above, it can be tested that if the scent of GMO foodstuffs come from raw materials or other genes, it would be haram or prohibited. This has become the fundamental of Islamic Law of MUI to certify food products made from GMO products.
In order to simplify the permission, the product labeled by Halal certification has to meet the standard of all Islamic Law, including GMO examination. The halal certification of GMO products distributed from certificates or labels that have been approved by the Institute for Drug, Cosmetics, and Food Studies - Indonesian Ulama Council (LPPOM) and the Food and Drug Supervisory Agency - Republic of Indonesia (BPOM). If GMO food has obtained a certificate and halal label from the official institution, an assessment and evaluation of the GMO products will be halal (Mahrus, 2014)
CONCLUSION
One of the major ways to terminate extreme poverty is an increase in agricultural activity. Genetically Modified Organism, or GMO, could potentially be a part of solving the poverty challenge (Whelan, 2016). Genetically Modified Food, or GMF, is a technology that can answer food problems in the world, especially in Indonesia. The GMF is controversial, many parties disagree, including the European Union, but Indonesia is still struggling with GMF consumption due to social issues of poverty and hunger. In Indonesia, it has also received a lot of food from GMF from foreign countries and has had research in terms of agricultural biotechnology, except that it is awaiting the government's decision to be allowed to grow by large numbers of farmers. Many positive effects from GMO such as an increase in the amount of food production and also the increase in the country's income. Thus, imported products will decline and the farmers will become more prosperous. Indeed, many people are worried about the negative impact of GMF products, but until now no one has been able to prove the negative impact of GM products.
In order to meet national food demand and reduce hunger, GMF is still supported by the government with terms and conditions of the Minister of Agriculture and Minister of Health of the Republic of Indonesia to maintain national food security under the supervision of the Food and Drug Supervisory Agency (BPOM), therefore GMO products in Indonesia remain within safe limits.
Genetically Modified Foods can make Indonesian people to have particularly high income. To compete with food produced by traditional agricultural systems, GMOs must be able to show that they are more profitable. Increasing the productivity of a food will increase the country's income and also more food will be produced to reduce hunger which is still happening a lot in some regions of Indonesia as well as stabilizing national food prices because of stable food stocks.
REFERENCES
Adduci, G. (2014, August 7th) GM Crops and Biosafety in South-East Asia: Singapore as Case Study. [Yearbook]
Anindyaputri, I. (2017, September 6th). Yang Perlu Anda Tahu Seputar Pangan Rekayasa Genetika. hellosehat.com. Retrieved from: https://hellosehat.com/hidup-sehat/nutrisi/pangan-rekayasa-genetika/
Central Bureau of Statistics (2017). Jumlah penduduk Indonesia dan pertumbuhannya, 2007-2016. Beritagar.id. Retrieved from: https://lokadata.beritagar.id/chart/preview/jumlah-penduduk-indonesia-dan-pertumbuhannya-2007-2016-1499396486
Central Bureau of Statistics (2018a, March 6th). 2020, Penduduk Indonesia Diproyeksi Mencapai 271 Juta Jiwa. databoks.katadata.co.id. Retrieved from: https://databoks.katadata.co.id/datapublish/2018/03/06/2020-penduduk-indonesia-diproyeksi-mencapai-271-juta-jiwa
Central Bureau of Statistics (2018b, August 21st). Impor Beras Indonesia SMT I 2018 Melonjak 755%. databoks.katadata.co.id. Retrieved from: https://databoks.katadata.co.id/datapublish/2018/08/21/impor-beras-indonesia-smt-i-2018-melonjak-755
Cornis, L. (2018). What are the political drivers for GMOs in developing countries?. Devex.com. Retrieved from https://www.devex.com/news/what-are-the-political-drivers-for-gmos-in-developing-countries-92091
Deswina P, Syarief R, Rachman LM and Herman M (2013). Policy Analysis of Sustainable GMO Management Using Decision Making Method in Indonesia. Adv Genet Eng 2:107. doi: 10.4172/2169-0111.1000107. Retrieved from https://www.omicsonline.org/open-access/policy-analysis-of-sustainable-gmo-management-using-decision-making-method-in-indonesia-2169-0111.1000107.php?aid=15258
Global Hunger Index (2018). Global Hunger Index Indonesia 2018. Global Hunger Index. Retrieved from https://www.globalhungerindex.org/indonesia.html
Indonesia Biosafety Clearing House (2012). Mencari Produk Unggul Lewat Rekayasa Genetik. IBCH. Retrieved from: http://indonesiabch.menlhk.go.id/mencari-produk-unggul-lewat-rekayasa-genetik/
Kompas (2018, April 3rd). 19,4 Juta Orang Indonesia Tidak Dapat Memenuhi Kebutuhan Pangan. Kompas.com. Retrieved from: https://ekonomi.kompas.com/read/2018/04/03/140000126/19-4-juta-orang-indonesia-tidak-dapat-memenuhi-kebutuhan-pangan
Naelufar, D. (2017, March 17th). Kelaparan di Indonesia Masih Level Serius. liputan6.com. Retrieved from: https://www.liputan6.com/news/read/2890073/kelaparan-di-indonesia-masih-level-serius
National Agency of Drug and Food Control (2017, April 27th). Klarifikasi Penjelasan tentang Isu Keamanan Pangan Produk Rekayasa Genetik. Badan POM. Retrieved from: https://www.pom.go.id/new/view/more/klarifikasi/50/Klarifikasi-Penjelasan-tentang-Isu-Keamanan-Pangan-Produk-Rekayasa-Genetik.html
National Agency of Drug and Food Control (2018). Peraturan badan pengawas obat dan makanan No. 6 Tahun 2018 tentang Pengawasan Pangan Produk Rekayasa Genetik. Ministry of Health. Jakarta: Indonesia
Mahrus (2014, July 2nd). Kontroversi Produk Rekayasa Genetika Yang Dikonsumsi Masyarakat. media.neliti.com. Retrieved from: https://media.neliti.com/media/publications/76423-ID-kontroversi-produk-rekayasa-genetika-yan.pdf
Saudale, V. (2018, October 18th). Produk Rekayasa Genetika Dukung Ketahanan Pangan. beritasatu.com. Retrieved from: http://www.beritasatu.com/nasional/517304-produk-rekayasa-genetika-dukung-ketahanan-pangan.html
Schnurr, M.A., Smyth, S.J. (2016). Can Genetically Modified Crops Help the Poor? Options for Canada’s Foreign Policy. Genome Canada: Policy Brief 12 April 2016. Retrieved from: https://www.genomecanada.ca/sites/genomecanada/files/genome_16-136_gps_policybrief_on_gmo_crops_brief_12_e_web.pdf
SourceWatch (2012, June 8th). USAID Promotion of Agricultural Biotechnology. sourcewatch.org. Retrieved from: https://www.sourcewatch.org/index.php/USAID_Promotion_of_Agricultural_Biotechnology
Sparringa, R. (2010). Current Regulatory Perspectives on GM Food in Indonesia. [Presentation] ILSI Region Seminar on” Science and Regulatory Perspectives on Stacked Events in Genetically Modified Crops”. Jakarta, 22-23 September 2010.
Sumarto (2017, October 28th). Seputar Pangan Rekayasa Genetika. Foodforkids.co.id. Retrieved from: http://foodforkids.co.id/post/579/2017-10-28/gizi/Seputar-Pangan-Rekayasa-Genetika
USDA Foreign Agricultural Service (2015). Indonesia Agricultural Biotechnology Annual 2015. Global Agricultural Information Network. GAIN Report Number: 1526. Retrieved from https://gain.fas.usda.gov/recent%20gain%20publications/agricultural%20biotechnology%20annual_jakarta_indonesia_7-14-2015.pdf
Whelan, K. (2016, April 28th). How GMOs Could Potentially End Poverty and Hunger in Africa. The Borgen Project. Retrieved from: https://borgenproject.org/gmos-potentially-end-poverty-hunger-africa/
Yuniar, N. (2014, November 12th). Bioteknologi bisa kurangi impor pangan. antaranews.com. Retrieved from: https://www.antaranews.com/berita/463928/bioteknologi-bisa-kurangi-impor-pangan.
|
Date submitted: Nov. 4, 2018
Reviewed, edited and uploaded: Dec. 10, 2018
|


The Controversial Case Study: Genetically Modified Food in Indonesia
INTRODUCTION
Genetically Modified Organism is an alternative way to improve both the quality and the quantity of agricultural products. In developing nations across the globe, governments are grappling with questions of what role, if any, genetically modified organisms should play in helping address a range of themes and issues: agriculture; nutrition; and climate challenges. (Cornis, 2018). The outputs of policy making in Indonesia is to ensure consumer protection by food labeling of GMO products. Indonesia has significant capacity to promulgate but limited capability to enforce regulations with respect to biosafety of GE products (USDA Foreign Agricultural Service, 2015). Indonesia’s regulations on GMO itself is still dependent on worldwide statement. This study aims to provide information on how Indonesian regulations address issues of GMO and how controversies occur within the Indonesian social sphere towards GM food consumption. The controversies and issues that will be discussed are around the environment, safety of consumption, biosafety, health impacts, cultural and religious issues.
Genetically Modified Food Defined
Genetically Modified Food (GMF) products are foods produced or that use raw materials, food additives, and/or other materials produced, from the genetic engineering process (National Agency of Drug and Food Control, 2018). Genetic engineering is defined as a biotechnology technique that is carried out by moving genes from one living creature to another. The transfer of the gene is referred to as GMO. Gene transfer can occur in the same or different species, for example from microorganism genes to plants or animals. The purpose of genetic engineering is to produce living things in the form of plants, animals, or microorganisms/microorganisms that have certain properties which are beneficial to humans. (Sumarto, 2017). The benefits of Genetic Engineering or GMO products are to improve the quality of plants so that plants are resistant to pests and diseases, resistant to drought stress, high salt content, frost resistant, and help improve the quality of nutrient content.
GMF in Indonesia
GMF is one of the results of a breakthrough in the application of biotechnology to increase food production. In Indonesia, many of these products have been mainly imported fruits and meat, as well as imported vegetables and imported food products. Most products containing GMO are vegetable and fruit products such as apples, oranges, bananas, and many more.
Indonesia still has not succeeded in developing GMO plants. All over the world, the development of genetically engineered food has been more advanced and is increasingly widespread. The United States is one country that has used transgenic seeds such as corn, tomatoes, potatoes, and papaya. (Anindyaputri, 2017).
The social problems and challenges faced in the circulation of GMF products, especially in Indonesia, are plants that have to do limited field testing and require a long time so that the process of implementing GMF products becomes long. Government regulations that are still unclear can trigger public confusion about the safety of consuming GM food products. Further research studies on GM products are still continuing but do not provide significant results, especially directly to the community. However, GM products have been circulating and most of the Indonesian population have consumed them. This became viral and controversial because many studies did not agree with GMO products. Various considerations such as the national economy, national food self-sufficiency and the government's mission to stabilize food prices nationally are still supporting factors for GMO products in Indonesia.
Significant increase in Indonesia’s population
The population in Indonesia has increased rapidly since a few years ago (refer to Fig. 1). Based on the projection of the National Development Planning Agency, the Central Statistics Agency, and the United Population Fund, Indonesia's population will reach 271 million by 2020. (Central Bureau of Statistics, 2018a). This has resulted in increased consumer demand for staple foods, while domestic food production is unable to support consumer demand.
Fig. 1. The Growth of Indonesia's population from 2007 to 2016 (Central Bureau of Statistics, 2017)
Instability of national food production
Domestically, many food productions are hampered by very erratic climate such as droughts or floods that eventually reduce food production in Indonesia. According to the Association of Indonesian Seed and Farm Technology Banks, in 2018, the current drought has the potential to reduce production potential by up to 60%. Unstable rice production, from January to March 2017, reaching 15.6 million tons. The details, production in January amounted to 2.8 million tons, February 5.4 million tons, and March 7.4 million tons. (Warta Ekonomi, 2018). As a result, Indonesia relies on imports of agricultural products to meet national needs. This also has a direct impact on the instability of rice prices every month.
Based on data from the Central Bureau of Rice Import Statistics up to the first semester of 2018, it has reached 1.12 million tons, which means it has jumped 755% compared to the first semester of 2017. Similarly, the value of rice imports in the first six months of this year surged more than 1600% to US$ 524.3 million.
During the second quarter of this year, rice imports reached 736,000 tons, up to 91.84% from the previous quarter and also jumped 765% compared to the same quarter last year. (Central Bureau of Statistics, 2018b).
Fig. 2. Indonesia’s rice import history 2015-2018. (Central Bureau of Statistics, 2018b)
Legal aspects of GMF in Indonesia
Indonesia’s regulation on GMF
Genetic modifying technology has been developed in Indonesia since the 1990s and as a result of new technology, is necessary to manage the product settings to prevent some causes like bad influences on humans, animals and the environment, especially its effect on overall biodiversity. The regulatory and management of Indonesia’s biological safety have been established by Governmental Regulation No. 21 of 2005 about GMO Biosafety and Presidential Regulation No. 39 of 2010 about Commission of Biosafety of GMO, which provide recommendations to the ministries and involved agencies related in the prerelease of GMO. (Deswina, et al, 2013).
The national competent authority for GM products
Table 1. The national competent authority list for GM Products (USDA Foreign Agricultural Service, 2015) (as cited in Indonesia Biosafety Clearing House, 2010; FAS, 2012)
GMF product distribution regulation in Indonesia
The Biosafety Commission is responsible for giving recommendations to the Minister and Head of Non-Ministry Government Institutions, authorized to prepare and determine policies and issue PRG biosafety certificates.
In the food business chain, anyone who produces or imports GMF products such retailers and distributors for food trading purpose must fulfill food safety, quality, and nutritional requirements in accordance with the legislation standards. In food business those who produce GM food domestically and/or import GM food for trading in retail packaging must include labels in accordance with the provisions of the legislation.
Points of consideration in Genetic Modified Food in Indonesia: (Indonesia Biosafety Clearing House, 2012)
Indonesia has regulated the circulation of GMF products in the domestic market. Before being distributed for public consumption, GM product foods must be reviewed and examined. This policy started since 1996 when the enactment of Law No. 7 the Year of 1996 concerning Food currently being revised into Law No. 18 the Year of 2012 concerning Food. In addition, Indonesia has ratified Cartagena Protocol on Bio-Safety to the Convention on Biological Diversity to become Law No. 21 the Year of 2004 concerning the Ratification of Cartagena Protocol on Bio-Safety to The Convention on Biological Diversity which applies the precautionary approach in the handling of GMOs. (National Agency of Drug and Food Control, 2017).
To implement this policy, the Government has compiled legislation related to the security assessment of GMF products, namely:
The Indonesian Ulama Council has issued an Islamic Law (fatwa) regarding PRG food through the Fatwa of the Indonesian Ulama Council Number 35 the Year of 2013 concerning Genetic Engineering Organism and its products.
International policy background
The United States supports the use of GMOs as part of the strategy to achieve its agricultural development goals. In 2002, USAID launched the Collaborative Agricultural Biotechnology Initiative (CABIO) “to promote developing country access to and management of new scientific tools such as biotechnology for improving agriculture productivity, environmental sustainability and nutrition” (U.S. Department of State and USAID 2005: 191). American will continue to fund biotechnology research and policy activities. This will include a shift from biotechnology research to field trials in Africa and Asia. (Sourcewatch, 2012)
Meanwhile, the European Union’s, Principle 15 of the 1992 Rio Declaration, EU doubts around GMOs stem from a broader interpretation of the precautionary principle, in which GMOs are viewed as foundationally different from previous techniques of selective and mutagenic breeding. Within this act the assessment of non-safety concerns is mandatory, and “can include any health and environmental consequences in the countries in which the crops are grown, notably developing countries. (Schnurr and Smyth, 2016)
In the Association of Southeast Asian Nations ASEAN countries, many still do not have the capacity to develop biosafety clearing-house mechanisms. Moreover, the policy on the national biosafety legal framework is vitally important and should be set as the first priority and be followed by a clear-cut plan of implementation for all the countries in the region. The ASEAN Ministers of Agriculture and Forestry approved the proposal of collaboration with the International Life Sciences Institute (ILSI) to conduct Training Workshop on Safety and Risk Assessment of Agriculture-Related GMOs annually.
Singapore is the only country to step out when describing the situation of agro-biotechnology in Southeast Asia. Singapore’s open import agriculture-related GM organisms is required to submit an application under specified Singaporean standard, which forwards it to the Subcommittee on the Release of Agriculture-Related GMOs. (Adduci, 2014).
Controversy and social issues of GMF in Indonesia
Until now, the controversy over genetically engineered food is still continuing, while GM products continue to be spread throughout almost the entire world and consumed by so many animals and humans.
Controversy began when according to the teaching staff at the Department of Food Science and Technology, Faculty of Agricultural Technology IPB, the negative law about GMF was incorrect, because every GMF variety before being released to the market must go through the testing section, namely: from structural tests, toxicity, testing to animals, released to a limited group, then marketed. When a variation has been released to the community, the Food Safety Commission of the Ministry of Agriculture conducts research and testing in the field.
What is meant by the Alliance Coordinator for Prosperous Villages, the Government of Indonesia regarding GMF products from abroad is safe, because foreign parties have conducted studies using resources and products that are in accordance with PRG that have the same substance, are not feasible towards varieties. The different statements are suggested to be addressed by the government by conducting research on GMF products, and publishing the results so that this anxiety and controversy are not prolonged.
Controversy over genetic engineering products consumed by communities
The existing domestic food products have not been able to overcome the problem of food shortages, and this has become a challenge for agricultural development in Indonesia. Many products that have been marketed are GMO products, but unfortunately, all GMO products in the market are not given clear information, therefore the most prominent problem still causing controversy in the community is if it is safe for GMO products to control the current market for consumption?
Pro GMO groups argue that there is unlimited potential in genetic engineering that is useful for reducing pesticide use, overcoming food shortages, and producing more nutritious foods and medicines. Groups that are against or refuse to think that food products and GMO drugs are not believed to be safe for consumption because they still cause various negative impacts on health and the environment. Another negative impact for farmers, in particular, is very detrimental to them because non-GMO farmers are unable to increase productivity and become more profitable.
The following describes the community controversy regarding the acceptance and use of GMO products in the fields of agriculture, environment, health, religion, culture, and ethics.
GMO controversy in agriculture and the environment
Positive:
In the long term this GMO plant will change the structure and texture of the soil which will have an impact on the quantity and quality of crop production. The positive impact of plants that are capable of producing substances that can eradicate weeds is to reduce costs because they do not need to buy herbicides which are relatively expensive for farmers.
Negative:
Genetically engineered plants have the potential to damage the balance of the surrounding environment. Pests and plant diseases will run into conventional fields so that inevitably, these farmers have to switch to using GMO crops which are relatively expensive. Environmentalists worry that GMO crops will pose environmental risks when they are widely cultivated. Another problem that is expected to emerge is the killing of other living creatures such as butterfly larvae, which in turn are feared because extinction of butterflies as a result of GMO residues is toxic.
GMO controversy in the health sector
Positive:
The production of GMO drugs such as insulin, monoclonal antibodies, anti-allergies, anti-cancer drugs and many other drugs to cure disease sharing has been felt by the community as reported by Singh et al. (2006). The ability to express foreign genes using genetic engineering technology has opened up the option to produce a large number of commercial food products and medicines/pharmaceuticals essential for improving public health.
Negative:
Genetically engineered food is thought to be the cause of various diseases with the assumption that foreign genes might alter the nutritional value of food in unexpected ways that can reduce or increase some other nutrients and nutrients. Factors that need to be considered from the minimum information is that one must be careful in using GMOfood products. Nordlee et al. (1996) reported that Brazil nuts as one of the GMO products were withdrawn from circulation because they caused allergies to consumers. These allergic reactions are thought to be caused by the modification of certain genes.
GMO controversy in the fields of religion, culture, and ethics
Muslim community groups in Indonesia as the majority group have provisions that require the food consumed is halal and good (halal toyyiban), so it is very important to include information about the content of food products and medicines produced by GMOs although it is not easy to trace the contents. The GMO requires a clear mechanism for tracking and monitoring GMO content that is widely circulated. Also, very important aspect is the inclusion of halal certificates issued by the Institute for the Study and Supervision of Medicines and Food of the Indonesian Ulema Council (LP POM MUI) so that the concern of the Muslim community in consuming GMO products is not developing and unsettling.
Food Safety Based on Legal Basis from National Agency of Drug and Food Control
Genetically Modified Food product requirements
Proteins that are encoded in the transferred gene are not allergens;
The method of extermination is used if there is a deviation.
Indonesia’s nationalstandard food safety information
Substantial value
Determination of substantial equivalence of genetically modified food requires consideration of the characteristics of foodstuffs or processed products which include a of food composition, phenotype and metabolite properties, food processing factors with conventionally obtained food.
Food component composition
Analysis of the composition of the components of PRG food or its processed products carried out for macro nutrients; proximate analysis (crude fiber, carbohydrate ash, fat, protein), fatty acids, amino acids and micronutrients (minerals, vitamins) and analysis of other components found in food that are deemed necessary
Phenotype properties
Metabolites
This food safety assessment requires an investigation of residual levels and metabolites in food and an assessment of altered nutrient profiles. If changes are identified in residual or metabolite levels, it must consider the potential impact on human health using conventional procedures to determine the safety of these metabolites or residues.
Food Processing
The potential influence of the food processing process on PRG food must also be taken into account. For example, there can be changes in endogenous toxicity and bioavailability of nutrients.
Changes in nutritional value
GM food products that are intentionally increased in nutritional value (for example with the presence of pro-vitamin A in rice and mustard), an assessment of the nutritional value of the GMF product must be carried out. Information about the consumption patterns of food and its processed products must use a similar approach.
Assessment of the nutritional value of GMF products. Information on the consumption patterns of processed foods must use a substantial similarity approach to conventional food consumption. The potential for undesirable effects can be detected using the approach to food use at the highest consumption level. Requirements for physiological characteristics and metabolites in special population groups such as infants, children, pregnant and lactating women, the elderly and those suffering from chronic diseases or those with damaged immune system needs attention.
Allergenicity
Food allergen city is a side-effect reaction that involves the immune system, namely increasing immunoglobulin E (IgE) in individuals who are very sensitive to special substances contained in food or food components, most food allergens are proteins but can also be hapten (small molecules that are antigen and cause allergies). Assessment of allergenic potential was carried out to assess the allergenicity of GMF products.
Toxicity
Information on PRG food toxicity testing covers at least acute toxicity to new proteins and sub-chronic toxicity to food. In vitro nucleic acid techniques allow the insertion of DNA that can produce a synthesis of new substances in plants. These new substances can be food components, such as protein, fat, carbohydrates and new vitamins in GMOs. The new substance can also be a new metabolite resulting from enzyme activity and the expression of inserted DNA.
Indonesia is still in serious hunger and poverty rates
In the 2018 Global Hunger Index, Indonesia ranks 73rd out of 119 qualifying countries. With a score of 21.9, Indonesia suffers from a level of hunger that is serious. (Global Hunger Index, 2018). More than 19 million Indonesians are still facing malnutrition. The data shown 2 to 3 children for every 100 children die before they are 5 years old. (Naelufar, 2017)
The Indonesian government claimed to overcome the problem of food sufficiency by increasing supply through increasing rice production and developing higher value crops. However, this strategy is proved to be ineffective because even though Indonesia experienced good economic growth, there were still 19.4 million citizens who could not meet their daily food needs. (Kompas, 2018).
GMO able to reduce dependence on imports and increase domestic food production
The use of biotechnology in agriculture can increase the productivity of food crops so that Indonesia can reduce imports (Yuniar, 2018). Indonesia has researched several genetically engineered plants that can increase farmer productivity because it has pest and disease resistance, one of which is GMF sugar cane event NXI-4T which is the world's first biotech sugar cane. Not only does it have pest and disease resistance, but also GMF is able to improve the quality of food products in Indonesia. Thus, it is not only possible to reduce food imports and also is able to reduce hunger rates in Indonesia.
According to the Professor Emeritus of Economics, Bogor Agricultural University (IPB), Prof. Dr. Parulian Hutagaol, revealed, genetic engineering techniques on food were first developed to answer various problems such as food security and climate change. (Saudale, 2018). Unfortunately, Indonesia still has concerns about biotech crops that disrupt the environment. As a result, environmental, food and feed tests at the government level take a short time.
The holiness of GMF based on Islamic law in Indonesia
A ruling on a point of Islamic law of the Indonesian Ulama Council (MUI) No 35 the Year of 2013 states that genetic engineering products are permitted (mubah) and under conditions of the nature of change genetic engineering products are useful for good reason, are harmless to environmental, do not apply human body genes or human parts. Based on the Islamic law above, it can be tested that if the scent of GMO foodstuffs come from raw materials or other genes, it would be haram or prohibited. This has become the fundamental of Islamic Law of MUI to certify food products made from GMO products.
In order to simplify the permission, the product labeled by Halal certification has to meet the standard of all Islamic Law, including GMO examination. The halal certification of GMO products distributed from certificates or labels that have been approved by the Institute for Drug, Cosmetics, and Food Studies - Indonesian Ulama Council (LPPOM) and the Food and Drug Supervisory Agency - Republic of Indonesia (BPOM). If GMO food has obtained a certificate and halal label from the official institution, an assessment and evaluation of the GMO products will be halal (Mahrus, 2014)
CONCLUSION
One of the major ways to terminate extreme poverty is an increase in agricultural activity. Genetically Modified Organism, or GMO, could potentially be a part of solving the poverty challenge (Whelan, 2016). Genetically Modified Food, or GMF, is a technology that can answer food problems in the world, especially in Indonesia. The GMF is controversial, many parties disagree, including the European Union, but Indonesia is still struggling with GMF consumption due to social issues of poverty and hunger. In Indonesia, it has also received a lot of food from GMF from foreign countries and has had research in terms of agricultural biotechnology, except that it is awaiting the government's decision to be allowed to grow by large numbers of farmers. Many positive effects from GMO such as an increase in the amount of food production and also the increase in the country's income. Thus, imported products will decline and the farmers will become more prosperous. Indeed, many people are worried about the negative impact of GMF products, but until now no one has been able to prove the negative impact of GM products.
In order to meet national food demand and reduce hunger, GMF is still supported by the government with terms and conditions of the Minister of Agriculture and Minister of Health of the Republic of Indonesia to maintain national food security under the supervision of the Food and Drug Supervisory Agency (BPOM), therefore GMO products in Indonesia remain within safe limits.
Genetically Modified Foods can make Indonesian people to have particularly high income. To compete with food produced by traditional agricultural systems, GMOs must be able to show that they are more profitable. Increasing the productivity of a food will increase the country's income and also more food will be produced to reduce hunger which is still happening a lot in some regions of Indonesia as well as stabilizing national food prices because of stable food stocks.
REFERENCES
Adduci, G. (2014, August 7th) GM Crops and Biosafety in South-East Asia: Singapore as Case Study. [Yearbook]
Anindyaputri, I. (2017, September 6th). Yang Perlu Anda Tahu Seputar Pangan Rekayasa Genetika. hellosehat.com. Retrieved from: https://hellosehat.com/hidup-sehat/nutrisi/pangan-rekayasa-genetika/
Central Bureau of Statistics (2017). Jumlah penduduk Indonesia dan pertumbuhannya, 2007-2016. Beritagar.id. Retrieved from: https://lokadata.beritagar.id/chart/preview/jumlah-penduduk-indonesia-dan-pertumbuhannya-2007-2016-1499396486
Central Bureau of Statistics (2018a, March 6th). 2020, Penduduk Indonesia Diproyeksi Mencapai 271 Juta Jiwa. databoks.katadata.co.id. Retrieved from: https://databoks.katadata.co.id/datapublish/2018/03/06/2020-penduduk-indonesia-diproyeksi-mencapai-271-juta-jiwa
Central Bureau of Statistics (2018b, August 21st). Impor Beras Indonesia SMT I 2018 Melonjak 755%. databoks.katadata.co.id. Retrieved from: https://databoks.katadata.co.id/datapublish/2018/08/21/impor-beras-indonesia-smt-i-2018-melonjak-755
Cornis, L. (2018). What are the political drivers for GMOs in developing countries?. Devex.com. Retrieved from https://www.devex.com/news/what-are-the-political-drivers-for-gmos-in-developing-countries-92091
Deswina P, Syarief R, Rachman LM and Herman M (2013). Policy Analysis of Sustainable GMO Management Using Decision Making Method in Indonesia. Adv Genet Eng 2:107. doi: 10.4172/2169-0111.1000107. Retrieved from https://www.omicsonline.org/open-access/policy-analysis-of-sustainable-gmo-management-using-decision-making-method-in-indonesia-2169-0111.1000107.php?aid=15258
Global Hunger Index (2018). Global Hunger Index Indonesia 2018. Global Hunger Index. Retrieved from https://www.globalhungerindex.org/indonesia.html
Indonesia Biosafety Clearing House (2012). Mencari Produk Unggul Lewat Rekayasa Genetik. IBCH. Retrieved from: http://indonesiabch.menlhk.go.id/mencari-produk-unggul-lewat-rekayasa-genetik/
Kompas (2018, April 3rd). 19,4 Juta Orang Indonesia Tidak Dapat Memenuhi Kebutuhan Pangan. Kompas.com. Retrieved from: https://ekonomi.kompas.com/read/2018/04/03/140000126/19-4-juta-orang-indonesia-tidak-dapat-memenuhi-kebutuhan-pangan
Naelufar, D. (2017, March 17th). Kelaparan di Indonesia Masih Level Serius. liputan6.com. Retrieved from: https://www.liputan6.com/news/read/2890073/kelaparan-di-indonesia-masih-level-serius
National Agency of Drug and Food Control (2017, April 27th). Klarifikasi Penjelasan tentang Isu Keamanan Pangan Produk Rekayasa Genetik. Badan POM. Retrieved from: https://www.pom.go.id/new/view/more/klarifikasi/50/Klarifikasi-Penjelasan-tentang-Isu-Keamanan-Pangan-Produk-Rekayasa-Genetik.html
National Agency of Drug and Food Control (2018). Peraturan badan pengawas obat dan makanan No. 6 Tahun 2018 tentang Pengawasan Pangan Produk Rekayasa Genetik. Ministry of Health. Jakarta: Indonesia
Mahrus (2014, July 2nd). Kontroversi Produk Rekayasa Genetika Yang Dikonsumsi Masyarakat. media.neliti.com. Retrieved from: https://media.neliti.com/media/publications/76423-ID-kontroversi-produk-rekayasa-genetika-yan.pdf
Saudale, V. (2018, October 18th). Produk Rekayasa Genetika Dukung Ketahanan Pangan. beritasatu.com. Retrieved from: http://www.beritasatu.com/nasional/517304-produk-rekayasa-genetika-dukung-ketahanan-pangan.html
Schnurr, M.A., Smyth, S.J. (2016). Can Genetically Modified Crops Help the Poor? Options for Canada’s Foreign Policy. Genome Canada: Policy Brief 12 April 2016. Retrieved from: https://www.genomecanada.ca/sites/genomecanada/files/genome_16-136_gps_policybrief_on_gmo_crops_brief_12_e_web.pdf
SourceWatch (2012, June 8th). USAID Promotion of Agricultural Biotechnology. sourcewatch.org. Retrieved from: https://www.sourcewatch.org/index.php/USAID_Promotion_of_Agricultural_Biotechnology
Sparringa, R. (2010). Current Regulatory Perspectives on GM Food in Indonesia. [Presentation] ILSI Region Seminar on” Science and Regulatory Perspectives on Stacked Events in Genetically Modified Crops”. Jakarta, 22-23 September 2010.
Sumarto (2017, October 28th). Seputar Pangan Rekayasa Genetika. Foodforkids.co.id. Retrieved from: http://foodforkids.co.id/post/579/2017-10-28/gizi/Seputar-Pangan-Rekayasa-Genetika
USDA Foreign Agricultural Service (2015). Indonesia Agricultural Biotechnology Annual 2015. Global Agricultural Information Network. GAIN Report Number: 1526. Retrieved from https://gain.fas.usda.gov/recent%20gain%20publications/agricultural%20biotechnology%20annual_jakarta_indonesia_7-14-2015.pdf
Whelan, K. (2016, April 28th). How GMOs Could Potentially End Poverty and Hunger in Africa. The Borgen Project. Retrieved from: https://borgenproject.org/gmos-potentially-end-poverty-hunger-africa/
Yuniar, N. (2014, November 12th). Bioteknologi bisa kurangi impor pangan. antaranews.com. Retrieved from: https://www.antaranews.com/berita/463928/bioteknologi-bisa-kurangi-impor-pangan.
Date submitted: Nov. 4, 2018
Reviewed, edited and uploaded: Dec. 10, 2018