Introduction
Gene recombination technology has generated both expectation and anxiety among its many followers. On the one hand, this new technology demonstrates a potential for increasing agricultural productivity. On the other hand, it enhances the risk of damaging the natural environment and consumers’health. The reduction of such risks is one of the primary goals of the Japanese government’s food policy. This paper describes the Japanese system for food safety assessment and labeling regulations for genetically modified (GM) crops, defined as varieties of plants created by using the technology of gene recombination, and GM foods, defined as fresh and processed foods produced from GM crops.
Assessment Methodology
Safety assessment of GM crops
The protection of biological diversity implies that GM crops should not be allowed to prevail on native varieties of organisms and microorganisms. The international framework for the protection of biodiversity is the Cartagena Protocol on Biosafety (CPB), which was adopted in 2000 as a supplementary agreement to the Convention on Biological Diversity. In 2003, Japan ratified the CPB and established the Act on the Conservation and Sustainable Use of Biological Diversity through Regulations on the Use of Living Modified Organisms, commonly called the Cartagena Act.
This regulation stipulates that the development of a new variety of GM crops be operated in two steps. The first step is laboratory research, carried on in a protected environment. Before proceeding to this step, the research plan needs to be approved by the Ministry of Education, Culture, Sports, Science and Technology.
After the new variety of GM crop is created and proved not to be harmful to biological diversity in the laboratory, experimental growing in isolated farmland is carried on. These areas are located on open-air fields separated from the surrounding land through a fence. The Ministry of Agriculture, Forestry and Fisheries and the Ministry of the Environment are responsible for the safety assessment of this second step. If the protection of biological diversity in the isolated farmland is evidenced, the new variety of GM crop is allowed to be grown in ordinary farms.
Safety assessment of GM foods
Even though the introduction of a GM crop respects biodiversity, it does not mean that the new variety of GM crop is safe for eating. Before a new type of GM food (including fresh foods) goes to the market, it needs to go through a food safety assessment from the Ministry of Health, Labour and Welfare based on the Food Sanitation Act. There are two critical requirements. First, compared with existing foods, the new type of GM food should not present significant differences in the physical and nutritional characteristics, type, and quantity of naturally occurring poison, and way of eating. Second, the protein from the gene recombination technology should not be noxious, for instance, causing allergy.
Food labeling regulations for GM foods
Currently, the Japanese government approves eight types of GM crops, and 33 types of GM processed foods, as shown in Table 1. This means that both GM and GM-free foods coexist in the market.
When one of the commodities listed in Table 1 is sold in the market, the Food Labeling Act (FLA) requires the seller to label clearly whether the commodity is GM-free or not.1 To be described as GM-free, a commodity should be kept separate from GM crops and GM foods in all phases of the production and marketing process (i.e., seeding, harvesting, collecting, manufacturing, wholesaling, and retailing). This condition is known as “identity preserved handling.” However, in the production and marketing process, unintentional contamination can happen even if producers and merchandisers use the greatest caution. For example, in a corn starch factory, a machine may be utilized for various types of corn snacks. Even if a machine is carefully washed before being used for non-GM corn, a small amount of GM corn is likely to remain inside the machine. The FLA specifies that the process is regarded as identity preserved handling if the unintentional contamination rate is lower than 5%.
If only GM-free crops are used to produce a commodity under the identity preserved handling condition, the “GM-free” food label is optional for the sellers. If GM crops are used as ingredients to produce a commodity, the sellers are required to clearly report it on the food label. However, the FLA specifies that the sellers are allowed to omit an ingredient from the food label if such ingredient represents less than 5% of the total weight of the final product and it is not among the top three ingredients. Therefore, food producers often use a GM crop as an ingredient without indicating it on the food label because its percentage in weight terms is small. For all food commodities not listed in Table 1, it is prohibited to report “GM-free” on the food label.
Discussion and Conclusions
The Japanese labeling policy for GM foods
Some experts of the food industry pointed out the three most important issues with the Japanese labeling policy for GM foods.2
First, compared with other developed countries, Japan allows one of the highest upper limits for unintentional contamination. Critics argue that, following the EU’s standards, the Japanese government should reduce that limit to 0.9%. Second, considering the high concerns of consumers for food safety, GM ingredients should be indicated even if they amount to less than 5% of the weight of the final product and do not figure in the top three ingredients. Third, some corn and soy products are excluded from the list in Table 1. A typical example is soy sauce. The Japanese government clarified that, since the genes of soybeans are totally broken up in the production process of soy sauce, distinguishing between GM-free soybeans and genetically modified beans would be meaningless. It is hard to prove scientifically whether soy sauce contains GM soybeans. However, considering consumers’ anxiety about food safety, critics claim that the government should require soy sauce producers to report the use of a GM crop in the soy sauce production process.
Table 1. GM foods approved by the Japanese government
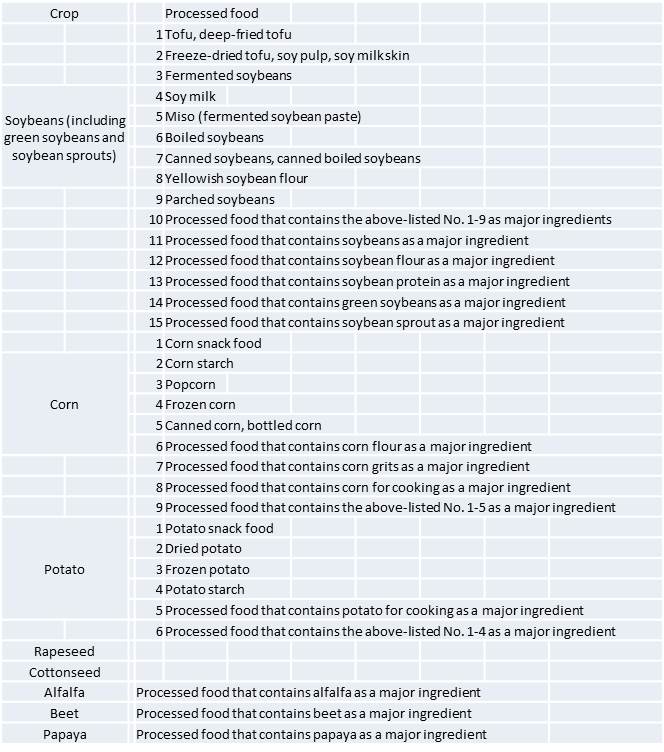
Source: Consumer Affairs Agency
Concluding remarks
As shown in Table 1, the Japanese government approves eight types of GM crops for commercial use. This means that there is no legal prohibition against growing these eight GM crops. However, fearing for Japanese consumers’ criticisms, very few farmers in Japan grow GM crops for commercial purposes.
The Japanese food labeling regulations for GM foods are not particularly strict. As a result, consumers often purchase food commodities produced with GM crops without being aware of it.
In addition, an overwhelming majority of Japanese livestock farmers rely on imported GM crops for feeding. Researchers estimate that Japan imports around 1.5 million tons of GM crops as ingredients of foods and feeds every year.3
The Japanese attitude towards GM crops seems to be characterized by the sharp contrast between a negligible level of domestic production of GM crops and a large import of GM crops. There are sharply different views on the safety and usefulness of GM crops and GM foods among researchers, producers, and consumers. Some claim that Japan should increase the domestic production of GM crops.4 Others assert that Japan should reduce its import of GM crops.5 The future development of the Japanese policy for GM crops and GM foods is uncertain.
Footnotes
- The Consumer Affairs Agency is responsible for the enforcement of the FLA.
- For example, see Kakita (2015).
- For example, see Tabei (2015).
- For example, see Yamanaka (2015).
- For example, see Tabei (2015). In 2016, the Consumer Union of Japan collected 197,879 signatures of people opposing to imports of GM foods and submitted them to the Japanese government.
References
Kakita, T., 2015. Issatsu de Wakaru Shokuhin Hyoji (A handbook of food labeling), Tokyo: Shogyokai.
Tabei, Y., 2015. Idenshi Kumikae Nosanbutsu (Genetically Modified Agricultural Products), in Matsuda, Tomoyoshi ed., Shokuhin no Anzen to Anshin (Food Safety and Security), Tokyo: Saiwaishobo.
Yamanaka, H., 2015. Shokuhin Hyoji no Wana (Tricks of Food Labeling), Tokyo: Chikuma Shobo.
|
Date submitted: Feb. 15, 2017
Reviewed, edited and uploaded: Feb. 21, 2017
|


The Japanese Policy for Genetically Modified Foods
Introduction
Gene recombination technology has generated both expectation and anxiety among its many followers. On the one hand, this new technology demonstrates a potential for increasing agricultural productivity. On the other hand, it enhances the risk of damaging the natural environment and consumers’health. The reduction of such risks is one of the primary goals of the Japanese government’s food policy. This paper describes the Japanese system for food safety assessment and labeling regulations for genetically modified (GM) crops, defined as varieties of plants created by using the technology of gene recombination, and GM foods, defined as fresh and processed foods produced from GM crops.
Assessment Methodology
Safety assessment of GM crops
The protection of biological diversity implies that GM crops should not be allowed to prevail on native varieties of organisms and microorganisms. The international framework for the protection of biodiversity is the Cartagena Protocol on Biosafety (CPB), which was adopted in 2000 as a supplementary agreement to the Convention on Biological Diversity. In 2003, Japan ratified the CPB and established the Act on the Conservation and Sustainable Use of Biological Diversity through Regulations on the Use of Living Modified Organisms, commonly called the Cartagena Act.
This regulation stipulates that the development of a new variety of GM crops be operated in two steps. The first step is laboratory research, carried on in a protected environment. Before proceeding to this step, the research plan needs to be approved by the Ministry of Education, Culture, Sports, Science and Technology.
After the new variety of GM crop is created and proved not to be harmful to biological diversity in the laboratory, experimental growing in isolated farmland is carried on. These areas are located on open-air fields separated from the surrounding land through a fence. The Ministry of Agriculture, Forestry and Fisheries and the Ministry of the Environment are responsible for the safety assessment of this second step. If the protection of biological diversity in the isolated farmland is evidenced, the new variety of GM crop is allowed to be grown in ordinary farms.
Safety assessment of GM foods
Even though the introduction of a GM crop respects biodiversity, it does not mean that the new variety of GM crop is safe for eating. Before a new type of GM food (including fresh foods) goes to the market, it needs to go through a food safety assessment from the Ministry of Health, Labour and Welfare based on the Food Sanitation Act. There are two critical requirements. First, compared with existing foods, the new type of GM food should not present significant differences in the physical and nutritional characteristics, type, and quantity of naturally occurring poison, and way of eating. Second, the protein from the gene recombination technology should not be noxious, for instance, causing allergy.
Food labeling regulations for GM foods
Currently, the Japanese government approves eight types of GM crops, and 33 types of GM processed foods, as shown in Table 1. This means that both GM and GM-free foods coexist in the market.
When one of the commodities listed in Table 1 is sold in the market, the Food Labeling Act (FLA) requires the seller to label clearly whether the commodity is GM-free or not.1 To be described as GM-free, a commodity should be kept separate from GM crops and GM foods in all phases of the production and marketing process (i.e., seeding, harvesting, collecting, manufacturing, wholesaling, and retailing). This condition is known as “identity preserved handling.” However, in the production and marketing process, unintentional contamination can happen even if producers and merchandisers use the greatest caution. For example, in a corn starch factory, a machine may be utilized for various types of corn snacks. Even if a machine is carefully washed before being used for non-GM corn, a small amount of GM corn is likely to remain inside the machine. The FLA specifies that the process is regarded as identity preserved handling if the unintentional contamination rate is lower than 5%.
If only GM-free crops are used to produce a commodity under the identity preserved handling condition, the “GM-free” food label is optional for the sellers. If GM crops are used as ingredients to produce a commodity, the sellers are required to clearly report it on the food label. However, the FLA specifies that the sellers are allowed to omit an ingredient from the food label if such ingredient represents less than 5% of the total weight of the final product and it is not among the top three ingredients. Therefore, food producers often use a GM crop as an ingredient without indicating it on the food label because its percentage in weight terms is small. For all food commodities not listed in Table 1, it is prohibited to report “GM-free” on the food label.
Discussion and Conclusions
The Japanese labeling policy for GM foods
Some experts of the food industry pointed out the three most important issues with the Japanese labeling policy for GM foods.2
First, compared with other developed countries, Japan allows one of the highest upper limits for unintentional contamination. Critics argue that, following the EU’s standards, the Japanese government should reduce that limit to 0.9%. Second, considering the high concerns of consumers for food safety, GM ingredients should be indicated even if they amount to less than 5% of the weight of the final product and do not figure in the top three ingredients. Third, some corn and soy products are excluded from the list in Table 1. A typical example is soy sauce. The Japanese government clarified that, since the genes of soybeans are totally broken up in the production process of soy sauce, distinguishing between GM-free soybeans and genetically modified beans would be meaningless. It is hard to prove scientifically whether soy sauce contains GM soybeans. However, considering consumers’ anxiety about food safety, critics claim that the government should require soy sauce producers to report the use of a GM crop in the soy sauce production process.
Table 1. GM foods approved by the Japanese government
Source: Consumer Affairs Agency
Concluding remarks
As shown in Table 1, the Japanese government approves eight types of GM crops for commercial use. This means that there is no legal prohibition against growing these eight GM crops. However, fearing for Japanese consumers’ criticisms, very few farmers in Japan grow GM crops for commercial purposes.
The Japanese food labeling regulations for GM foods are not particularly strict. As a result, consumers often purchase food commodities produced with GM crops without being aware of it.
In addition, an overwhelming majority of Japanese livestock farmers rely on imported GM crops for feeding. Researchers estimate that Japan imports around 1.5 million tons of GM crops as ingredients of foods and feeds every year.3
The Japanese attitude towards GM crops seems to be characterized by the sharp contrast between a negligible level of domestic production of GM crops and a large import of GM crops. There are sharply different views on the safety and usefulness of GM crops and GM foods among researchers, producers, and consumers. Some claim that Japan should increase the domestic production of GM crops.4 Others assert that Japan should reduce its import of GM crops.5 The future development of the Japanese policy for GM crops and GM foods is uncertain.
Footnotes
References
Kakita, T., 2015. Issatsu de Wakaru Shokuhin Hyoji (A handbook of food labeling), Tokyo: Shogyokai.
Tabei, Y., 2015. Idenshi Kumikae Nosanbutsu (Genetically Modified Agricultural Products), in Matsuda, Tomoyoshi ed., Shokuhin no Anzen to Anshin (Food Safety and Security), Tokyo: Saiwaishobo.
Yamanaka, H., 2015. Shokuhin Hyoji no Wana (Tricks of Food Labeling), Tokyo: Chikuma Shobo.
Date submitted: Feb. 15, 2017
Reviewed, edited and uploaded: Feb. 21, 2017