ABSTRACT
Soil-borne diseases are one of the factors that limit the productivity of agricultural crops in Indonesia. Soil-borne pathogens that predominantly attack various types of agricultural plants in Indonesia include Fusarium spp., Pythium spp., Phytophthora spp., Ralstonia solanacearum, Ganoderma boninense, and Rigidoporus microporus. Sometimes the losses caused by the attack of soil-borne pathogens are very high in economic terms. Management of controlling soil-borne diseases in Indonesia is by using organic fertilizers, both those which are enriched with beneficial microbes and minerals or not, the use of biological fertilizers including biopesticides, and improvement of cultivation systems that suppress the development of the soil-borne diseases. One potential that is being developed is research and development of endophyte dark septate fungi (DSE)-based on fertilizer technology to promote plant growth and production under biotic and abiotic stress conditions including the control of soil-borne diseases in Indonesia.
Keywords: Organic fertilizer, Bio-fertilizer, Bio-pestices, Soil-borne diseases, Dark septate endophytic fungi
INTRODUCTION
Fertile soil does not always indicate that the soil is healthy. The abundant pathogenic microbial population in that soil may cause a decline in the crop productivity although the soil is abundant with rich nutrients (Fiers et al., 2012; Frac et al., 2018). Soil-borne diseases are more restrictive in the production of many agricultural crops and contribute 10-20% of yield losses annually compared with seed-borne and air-borne diseases (Yuliar et al., 2015). For example, the resulting losses due to soil borne diseases caused by nematodes in plants around the world are estimated at US$ 80 billion per year (Price, 2000). The distribution of nematode attacks is not only in vegetables but also includes food crops and plantations such as Meloidogyne incognita, M. javanica, and M. arenaria which is the most destructive root-knot nematode to infect potato in Indonesia (Mutala’liah et al., 2018). Syafi'i et al. (2018) stated that the potato cyst nematode, Globodera rostochiensis, is increasingly widespread in potato plantations in Wonosobo and Banjarnegara, Central Java, Indonesia, and if not controlled it will extend to other parts of Indonesia.
Soil-borne pathogen bacteria, Ralstonia solanacearum, that causes bacterial wilt, has an extensively wide host, over 200 species (Schell, 2000; Yuliar et al., 2015) and still a major obstacle in many crop cultivation in Indonesia and induces a destructive economic impact (Kelman, 1998; Yuliar et al., 2015). This disease is included in the five main diseases in several countries and causes considerable loss of yield (20-45%), the quality of the seed produced is low and causes land contamination so that it cannot be planted for a long time (Mansfied et al., 2012; Yuliar et al., 2015). In Indonesia, R. solanacearum is the main factor that causes low yields of peanuts (Nugrahaeni, 2011). R. solanacearum attacks economically important plants, such as tomatoes, potatoes, tobacco, bananas, and peanuts (Hayward 1991; Elphinstone 2005; Hemelda et al., 2019). R. solanacearum has become an important pathogen in agriculture in Java, Indonesia (Hemelda et al., 2019).
Fusarium oxysporum is an important pathogen that attacks various economically valuable plants in Indonesia such as tomatoes, chilies, shallot, onion, and banana (Nasir et al., 1999; Nion and Toyota, 2008; Hwang and Ko, 2014; Supyani and Widadi, 2015; Pedai et al., 2015; Prihatna et al., 2018; Maryani et al., 2019). This pathogen can attack its host plants in all phases of growth (Cahyono, 2008; Mihajlović et al., 2017). In tomato plants, losses caused by the attack of these pathogens in Indonesia can reach 20-30% (Wibowo, 2007). Fusarium wilt caused by F. oxysporum is also a potential pathogen of chilies in Indonesia (Ali, 2006; Ferniah et al., 2018). Nasir et al. (1999) reported that Fusarium wilt on banana plants occurs in almost all parts of Indonesia from Aceh to Papua (Maryani et al., 2019). The impact of the Fusarium oxysporum attack on banana plants is the destruction of thousands of hectares of banana plantations, both commercial banana plantations and people's banana plantations (Riska and Hermanto, 2012).
Soil-borne diseases also attack the main crops of plantations in Indonesia such as rubber, oil palm and pepper. Soil-borne pathogen, Ridigoporus microporus attacks rubber plants in all phases of growth and causes huge economic losses in Indonesia (Kaewchai and Soytong, 2010; Sakpetch et al., 2018). Whereas in oil palm plants, a large economic loss is caused by the attack of soil-borne pathogen, Ganoderma boninense, which attacks both in the nursery phase and the plant phase to produce fruit and as a most serious disease in oil palm plantation in Indonesia (Darmono et al., 2000; Rees et al., 2012; Lisnawati et al., 2016; Paterson, 2019). In pepper plants, Phytophthora capsici, is a soil-borne pathogen that attacks pepper plantations in Lampung, Indonesia and gives great damage to people's pepper plantations (Manohara et al., 2004; Hendra et al., 2014).
Table 1. Some soil-borne pathogens and their host plant distribution and the diseases caused by these pathogens in Indonesia
|
No
|
Soil-borne pathogen
|
Host plants
|
Disease
|
|
Fungi
|
|
1
|
Fusarium spp.
|
Tomato, banan, shallot, chilli, melon, legume crops
|
Fusarium wilt, damping off
|
|
2
|
Pythium spp.
|
Cucumber
|
Damping off
|
|
3
|
Phythopthora spp.
|
Pepper, orange, cacao, tobacco, vegetables, legume crops
|
Phytophthora foot rot, blight and fruit rot
|
|
4
|
Ganoderma boninense
|
Oil palm
|
Basal stem rot
|
|
5
|
Rigidoporus microporus
|
Rubber
|
White root rot
|
|
Bakteri
|
|
1
|
Ralstonia solanacearum
|
Tomato, banana, potato, ginger, peanut
|
Bacterial wilt
|
|
Nematode
|
Food crops, horticultural crops and plantation crop
|
Root galls
|
Processed from various sources
Soil health in terms of soil biological aspects
The quality of soil biology in an agricultural land resource is an important component and one of the factors that determine the sustainability of the function of the land to support optimal and sustainable soil and plant productivity (Abawi and Widmer, 2000; Frac et al., 2018). The “sick soil” will cause plants that grow on that soil to be sick too, which will significantly decline plant productivity (Huang et al., 2006). The health aspects of the soil related to soil biological conditions is an important component that is often forgotten in determining the quality of agricultural land (Abawi and Widmer, 2000). The term sick soil is not only limited to marginal soils that are infertile due to the unavailability or lack of nutrients available that can be used by plants but also includes soils which have an abundance of soil pathogen population or severe outbreaks of soil-borne diseases (Huang et al., 2006). Texture, pH, organic matter, temperature and soil nutrients strongly influence the activity of soil-borne pathogens and soil health (Fiers et al., 2012).
Soil health refers to the capacity of the soil to maintain environmental quality, sustain biological productivity, and promote plant health (Frac et al., 2018; Haney et al., 2018). Current soil and plant health issues are no longer just concerns of plant disease scientists but become also the concern of soil scientists to develop sustainable soil health management which combines improved environmental quality, biological productivity and plant health (Frac et al., 2018). Soil-borne diseases have become one of the limiting factors for productivity in various types of plants, both horticulture, food and plantations (Mihajlovic et al., 2017; Katan, 2017). Soil-borne diseases can be latent because some pathogens that cause infectious diseases can live decades in the soil to interact again with host plants and survive in the soil from season to season (Nelson, 1982; Lucas, 2006; Fiers et al., 2012; Lecreck et al., 2014).
Recently, the control practices of soil-borne diseases in Indonesia use chemical pesticides such as fungicides and bactericides on a large scale. Under certain conditions or short-term, the use of pesticides will eradicate the target pathogen with great satisfaction to farmers, but useful microbes will also be eradicated so that the balance of the microflora and soil fauna is disrupted, in the long run there is a very severe attack and the emergence of resistant pathogens (Montesinos, 2003; Pimentel et al., 2007; Aktar et al., 2009). In addition, the use of pesticides causes soil pollution and the condition of the soil is getting sicker (Pimentel et al., 2007; Aktar et al., 2009). The problem of pest and plant disease resistance can occur due to improper use of pesticide compounds (Milus and Parsons, 1994). Therefore, technology is now being sought to improve soil health and control soil-borne diseases by using environmentally friendly active ingredients such as organic fertilizers (compost), biological fertilizers and biopesticides for the control of major soil-borne diseases in Indonesia based on conventional and molecular approaches.
Soil-borne disease management strategies: promoting the role of organic and biological fertilizers (plant growth promoter and bio-control/biopesticide) in improving soil health and controlling soil-borne diseases in Indonesia
Organic fertilizers such as compost is a complete source of macro and micro nutrients even in low quantities and can be used by plants to grow and produce (Setyorini et al., 2006) so that high yields of compost doses are needed to obtain adequate results. The presence of beneficial microbial community in compost can suppress pathogenic populations in the soil (Soekarno et al., 2013; Pugliese et al., 2014). Some microbial groups of antagonist bacteria (Bacillus spp., Pseudomonas spp.) and fungi (Trichoderma spp., Aspergillus spp., Penicillium spp.) are the most microbes found in compost (Tiquia and Michel, 2002; Ryckeboer et al., 2003). One of the environmentally friendly control strategies for soil-borne disease in vegetable crops is to use compost or other organic amendment, which results depend on the type of compost, application level and pathosystem (Bailey and Lazarovits, 2003; Pugliese et al., 2014).
The application of compost to increase organic carbon content in the soil will also increase beneficial soil microbial populations that suppress soil-borne pathogen (Noble and Coventry, 2005). Compost will stimulate soil microbial activity because it provides a substrate for microbes to active and become more dominant than pathogenic microbes (Pugliese et al., 2008). The addition of compost can increase the growth of soil microbes that are antagonistic to pathogens (Yulianti and Nidar, 1999). The results of the study of Carrise et al (2003) and Postma et al. (2003) showed that microbes from compost are able to suppress Pythium ultimum in cucumber and Verticillium biguttatum in sugar beets and tomatoes. The results of the research conducted by Soekarno et al. (2013) showed that the addition of compost enriched with PGPR bacteria (SR1L4 and SR2C3R1 bacteria), humic acid, and fulvic acid was able to increase the diversity of soil microbial populations in cucumber plant growth media and suppress Pythium sp attacks, causing damping off disease.
Table 2. Effect of growing media content on the diversity of soil microbial populations in cucumber plant growing media
|
Soil phase contions
|
Growing media content
|
Bacteria (cfu/ml)
|
Fungi (cfu/ml)
|
Actinomycetes (cfu/ml)
|
|
Before treatments
|
X
|
3.50.107
|
2.00.103 – 1.50.105
|
0
|
| |
Z
|
3.00.105 – 2.50.107
|
2.00.103
|
0
|
| |
|
|
|
|
|
After treatments
|
X
|
1.35.106 – 3.30.108
|
1.50.105
|
0
|
| |
Y
|
2.75.106 – 3.50.108
|
0.50.105 – 0.50.107
|
0
|
| |
Z
|
10.9.106 – 28.1.108
|
2.00.105
|
1.50.105
|
| |
AH
|
24.8.106 - 5,50.108
|
30.105 – 2.50.107
|
4.50.105 - 2.00.107
|
| |
AF
|
19.0.106 – 26.3.108
|
9.00.105 - 8.00.107
|
2.00.105 - 1.00.107
|
| |
AHF
|
10.3.106- 2.50.108
|
6.50.105 - 4.00.107
|
1.00.107
|
| |
SR1
|
29.6.106 – 7.05.108
|
29.5.105 – 4.50.107
|
3.50.105 – 0.50.107
|
| |
SR2
|
18.2.106- 1.05.108
|
14.0.105 – 2.50.107
|
7.50.105 - 2.00.107
|
| |
SR3
|
11.3.106 – 1.30.108
|
15.0.105 – 8.50.107
|
1.00.105 – 1.50.107
|
Note :
x: soil without compost, humic acid, fulvic acid, bacterium (negative control), y: sterilized soil without compost, humic acid, fulvic acid, bacterium (positive control), z: compost treatment AH : compost+humic acid, AF : compost+fulvic acid, AHF : compost+humic acid+fulvic acid,
SR1 : compost+bacterium SR1L4, SR2 : compost+bacterium isolate SR2C3R1, SR3 : compost+bacterium isolate SR1L4+bacterium isolate SR2C3R1
(Source : Soekarno et al., 2013)
Addition of organic matter especially compost can increase soil microbial activity because more energy sources are provided and soil conditions are made better for soil microbial activity and development (Bailey and Lazarovits, 2003; Pugliese et al., 2014). According to Campbell (1989) the use of organic fertilizers in addition to improving soil structure and providing nutrients, suppresses germination of pathogenic spores, causes lysis of pathogenic microbial cells, deactivates transient and permanent pathogen growth, supports non-activity -pathogen in providing nutrients and growth stimulating compounds for plants so as to improve the health of soil and plants. Surono et al. (2012) stated that compost enriched by humic acid and activator bacterium BX2 gives a positive effect on inhibiting infection of Fusarium sp. causing damping off in tomato plants and improves the agronomic potential of tomato plants. Based on research by Hendra et al. (2014) the use of coffee skin compost enriched with zeolite, humic acid, fulvic acid and microbial activator can suppress the attack of soil-borne pathogens Phythopthora capsici which causes rot of the stem at around 50% pepper.
In addition to organic fertilizers, there is another type of fertilizer that has a role in promoting plant growth and suppressing plant disease attacks, namely biofertilizer. Biofertilizer contains beneficial live organisms that function to facilitate the availability of nutrients in the soil for plants (Simanungkalit et al., 2006), to promote plant growth and to control plant disease (Surono and Narisawa, 2018). In addition to pathogenic microbes, in the soil, there are also various types of microbes that are beneficial for soil health. These microbes consist of bacteria, actinomycetes, fungi, and non-pathogenic nematodes. These beneficial soil microbes develop according to their soil habitat conditions. The functions of these soil microbes include nitrogen fixation, phosphate solubilizer, production of growth regulators and controlling diseases including soil-borne disease (Baum et al., 2015; El-Komy et al., 2015; Frac et al., 2018). Various beneficial soil microbes have been developed into biological fertilizer products that serve to support soil and plant productivity.
Formulations of various types of microbes to be used as biological fertilizer formulas are now rapidly being developed especially in Indonesia. Various products have been in the market with various kinds of active microbial contents. Generally, biofertilizer products developed in Indonesia are biofertilizers containing nitrogen-fixing bacteria, phosphate solubilizer, plant growth promoter and plant disease controllers, including soil-borne disease controller (Direktorat Pupuk dan Pestisida, 2015). For the most part, biofertilizer products are made from microbial active substances such as Bacillus, Pseudomonas, and Trichoderma (Direktorat Pupuk dan Pestisida, 2015).
Soekarno et al. (2014) stated that the use of Trichoderma active pellet formula is very effective in suppressing the growth of Pythium sp. causes of damping off disease. Surono et al. (2012) stated that the application of compost-enriched by bacterium BX2 and humic acid increased the population of soil microbes in growth media of tomato plants thus enhanced both soil and plant health. Based on the results of research by Soekarno et al. (2012) that selected bacterium B6 isolate-enriched compost applied to soil as a growing medium for peanut plants infested with Sclerotium rolfsii was able to control the incidence of damping off disease caused by that soil-borne pathogen in peanut plants by 93.75%. Safitri et al. (2012) stated that the application of endophytic bacteria was able to suppress Phytophthora foot rot disease from 51.95% to 95.63% in black pepper seedlings and the disease severity in ranged of 1,28%-29,35%. Endophytic bacteria isolated from the roots of oil palm plants can suppress the growth of G. boninense (Nasahi et al., 2016) and the compounds produced by endophytic bacteria are also able to inhibit the growth of G. boninense (Widiantini et al., 2018). Development of biological fertilizers should consider site-specific conditions with indigenous microbial resources that have been available in the field because the conditions of agricultural land in Indonesia have high diversity or heterogeneity (Subowo et al., 2013).
Research and development of dark septate endophytic fungi (DSE)-based fertilizer and pesticide for major soil-borne disease control in Indonesia
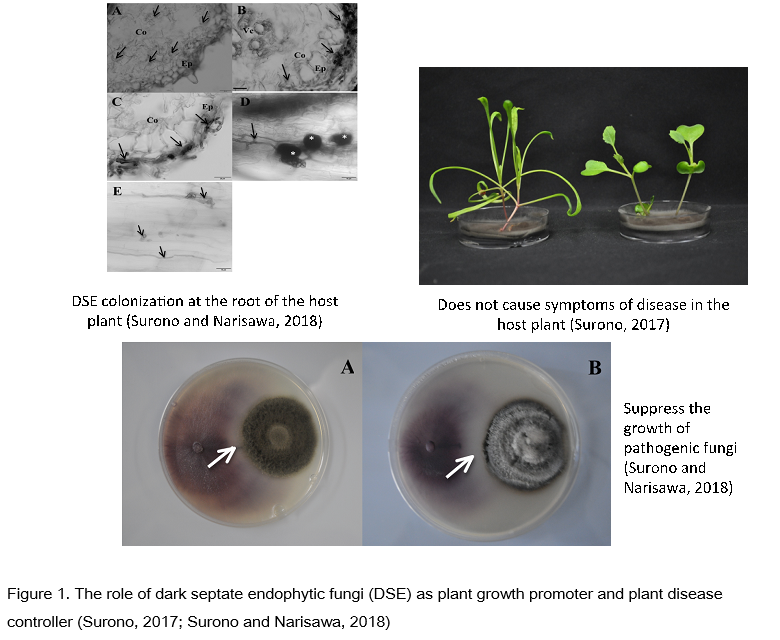
Surono and the team from the Indonesian Soil Research Institute (ISRI) and Bogor Agricultural University (IPB University) have researched and developed DSE isolated from various agroecosystems in Indonesia. DSE fungi have been isolated and selected both in laboratories, greenhouses and fields. Various types of DSE have been collected and examined for their benefits to stimulate the growth of various plants under biotic and abiotic stress conditions including for the control of soil-borne diseases such as Fusarium oxysporum f.sp. lycopersici in tomatoes (Zuhay et al., 2018), can suppress the development and attack of Ridigodoporus microporus in rubber plants (Surono et al, 2019, not yet published) both in the laboratory and in rubber plant nurseries and can suppress the growth of Ganoderma boninense in oil palm plants ( Surono et al, not yet published). The research conducted by Surono and Narisawa (2018) states that the endophyte dark septate fungus, Phialocephala fortinii, is capable of suppressing Fusarium oxysporum f.sp. asparagi on asparagus plants and increase the growth of asparagus in organic conditions (Surono and Narisawa, 2017). Not only suppresses soil-borne disease, DSE isolated by Surono et al. (2018) able to suppress blast disease caused by Phyricularia oryzae in rice plants. Research related to DSE fungal based on conventional and molecular approaches is underway.
Indonesian programs and achievements on the use of organic and biological fertilizers to improve soil and plant health in Indonesia
Soil-borne diseases are increasing everywhere in Indonesia, especially in the face of climate change, which now often occurs in an extreme way which affects the production of agricultural products, although the attack of the soil-borne disease still gets less attention compared to air-borne and seed-borne disease in the field (Yuliar et al., 2015; Syafi’i et al., 2018; Mutala’liah et al., 2018; Maryani et al., 2018; Hemelda et al., 2019). This often encourages farmers to use pesticides excessively without producing the dosage that should be given.
Based on the results of several studies that have been conducted (Surono et al., 2012; Soekarno et al., 2013; Soekarno et al., 2012; Safitri et al., 2012; Hendra et al., 2014), organic fertilizers, especially compost in addition to being used as a plant nutritional sources, can also be used to control soil-borne diseases such as damping off, basal stem rot, foot rot, and white root rot caused by soil-borne pathogens. Therefore, the use of organic fertilizers needs to be encouraged and increased in the use in the field by Indonesian farmers and agro-industry sectors. In addition to organic fertilizers, biological fertilizers which contain active ingredients are beneficial microbes especially use indigenous Indonesian microbes that can be synergistically with organic fertilizer utilized to support sustainable crop production. The Indonesian government has supported this program by implementing an organic fertilizer subsidy program and direct assistance to farmers in the form of organic and biological fertilizers, and equipment and machinery to support organic fertilizer production directly by farmers.
The use of organic and biological fertilizers can have an impact on environmental health. Various studies in the laboratories, greenhouses, and fields have been carried out by showing results that satisfy the positive influence in controlling the soil-borne disease by using organic and biological fertilizers. These potentials and opportunities require policy direction from the Government so that relevant stakeholders can develop technology in a targeted manner based on existing protocols so as not to harm the community, especially farmers as end users in the field. Through the Research Center and in synergy with the Observation Centers for Plant Pest and Disease Organisms under the Ministry of Agriculture and Local Government, environmentally friendly technologies for controlling soil-borne disease and other diseases are disseminated to Indonesian farmers through counseling, extension, and integrated agricultural pest and disease control schools.
CONCLUSION
Indonesian farmers' knowledge of benefits, how to use, and how to obtain organic fertilizers and biological fertilizers increase by training and field farmer school. Some farmer communities have practiced producing biofertilizers what they call local microorganisms (MOL), which are biological fertilizers that contain various types of microbes, in the cultivation of their plants even without clear quality control. Research on organic and biological fertilizers to control soil-borne pathogens both in the laboratory, greenhouse and field fields has shown satisfactory results. To speed up the process of spreading the use of organic and biological fertilizers without or combined between them to suppress soil-borne diseases, it needs to be developed, promoted and promoted through extension activities in the form of demonstration plots and socialization and training, workshops and seminars. The Indonesian Agency for Agricultural Research and Development (IAARD) in collaboration with the Local Government of Agriculture Office supported by the private sector increasingly improve demonstration plots, leaflets, booklets, and training as an active means of disseminating this program.
REFERENCES
Abawi, G.S., Widmer, T.L. 2000. Impact of soil health management practices on soilborne pathogens, nematodes and root diseases of vegetable crops. Applied Soil Ecology 15: 37–47.
Aktar, M.W., Sengupta, D., Chowdhury, A. 2009. Impact of pesticides use in agriculture: their benefits and hazards. Interdisc Toxicol. Vol. 2(1): 1–12. doi: 10.2478/v10102-009-0001-7.
Ali, M. 2006. Chili (Capsicum spp.) food chain analysis: Setting research priorities in Asia. Technical Bulletin No. 38. AVRDC - The World Vegetable Center, Taiwan.
Bailey, K.L., Lazarovits, G.. 2003. Suppressing soil-borne diseases with residue management and organic amendments. Soil & Tillage Research 72: 169–180.
Baum, C., El-Tohamy, W., and Gruda, N. 2015. Increasing the productivity and product quality of vegetable crops using arbuscular mycorrhizal fungi: a review. Sci. Hortic. 187, 131–141. doi: 10.1016/j.scienta.2015.03.002.
Cahyono, B. 2008. Tomat Usaha Tani dan Penanganan Pasca Panen. Kanisius: Yogyakarta.
Campbell, R., 1989. Biological Control of Microbial Plant Pathogens. 1st Edn., Cambridge University Press, Cambridge, ISBN: 0 521 34900 1.
Carisse, O., Bernier, J., Benhamou, N. 2003: Selection of biological agents from composts for control of damping-off of cucumber caused by Pythium ultimum. Plant Pathol. 25, 258-267.
Darmono, T. 2000. Ganoderma in oil palm in Indonesia: Current status and prospective use of antibodies for the detection of infection. In Ganoderma Diseases of Perennial Crops; Flood, J., Bridge, P.D., Holderness, M., Eds.; CABI Publishing: Wallingford, UK; pp. 249–266.
Direktorat Pupuk dan Pestisida, Direktorat Jenderal Prasarana dan Sarana Pertanian, Kementerian Pertanian Indonesia. 2015. Pupuk Terdaftar.
El-Komy, M. H., Saleh, A. A., Eranthodi, A., and Molan, Y. Y. 2015. Characterization of novel Trichoderma asperellum isolates to select effective biocontrol agents against tomato Fusarium wilt. Plant Pathol. J. 31, 50–60. doi: 10.5423/PPJ.OA.09.2014.0087.
Elphinstone JG. 2005. The current bacterial wilt situation: A global view. In: Allen C, Prior P, Hayward AC (eds) Bacterial Wilt Disease and the Ralstonia solanacearum Species Complex. American Phytopathological Society Press, Minnesota.
Fiers, Edel-Hermann, Chatot, Hingrat, Alabouvette, et al. 2012. Potato soil-borne diseases. A review. Agronomy for Sustainable Development, Springer Verlag/EDP Sciences/INRA, 32 (1), pp.93- 132. ff10.1007/s13593-011-0035-zff. ffhal-00930506.
Frac, M., Hannula, S.E., Bełka, M. and Jedryczka, M. 2018. Fungal Biodiversity and Their Role in Soil Health. Front. Microbiol. 9:707. doi: 10.3389/fmicb.2018.00707.
Haney, R.L., Haney, E.B., Smitha, D.R., Harmela, R.D., White, M.J. 2018. The soil health tool—Theory and initial broad-scale application. Applied Soil Ecology 125: 162–168.
Hayward, A.C. 1991. Biology and epidemiology of bacterial wilt caused by P. solanacearum. Ann. Rev. Phytopath. 29:67–87.
Hemelda NM, Safitri R, Suhandono S. 2019. Genetic diversity of Ralstonia solanacearum, A phytopathogenic bacterium infecting horticultural plants in Java, Indonesia. Biodiversitas 20: 364-372.
Hendra, J., Widodo, Soekarno, B.P.W., Bintoro, H.M., Manohara, D. 2014. Perspektif Pengembangan Kompos Bioaktivator untuk Pengendalian Phytophthora capsici pada Tanaman Lada (Piper nigrum). Jurnal Pengkajian dan Pengembangan Teknologi Pertanian Vol. 17, No.1,: 37-48.
Huang, H.C., Chou, C.H., Erickson, R.S. 2006. Soil sickness and its control. Allelopathy Journal 18(1): 1-21.
Hwang, S.C., Ko, W.H. 2014. Cavendish banana cultivars resistant to fusarium wilt acquired through somaclonal variation in Taiwan. Plant Dis. 88(6): 580-588. doi:10.1094/PDIS.2004.88.6.580.
Kaewchai, S., Soytong, K., 2010. Application of biofungicides against Rigidoporus microporus causing white root disease of rubber trees. Journal of Agricultural Technology 2010. 6(2), 349-363.
Katan, J. 2017. Diseases caused by soilborne pathogens: biology, management and challenges. Journal of Plant Pathology, 99 (2): 305-315.
Kelman, A. 1998. One hundred and one years of research on bacterial wilt, p. 1–5. In P.H. Prior, C. Allen and J. Elphinstone (ed.), Bacterial Wilt Disease: Molecular and Ecological Aspects. Springer, Heidelberg.
Leclerc, M, Dore´, T., Gilligan, C.A,. Lucas, P., Filipe, J.A.N. 2014. Estimating the Delay between Host Infection and Disease (Incubation Period) and Assessing Its Significance to the Epidemiology of Plant Diseases. PLoS ONE 9(1): e86568. doi:10.1371/journal.pone.0086568.
Lisnawita, Hanum, H, Tantawi, A.R. 2016. Survey of Basal Stem Rot Disease on Oil Palms (Elaeis guineensis Jacq.) in Kebun Bukit Kijang, North Sumatera, Indonesia. International Conference on Agricultural and Biological Sciences (ABS 2016), Earth and Environmental Science. 2016; (41)012007.
Lucas, P. 2006. Diseases caused by soil-borne pathogens. In: Cooke BM, Jones DG, Kaye B, editors. The Epidemiology of Plant Diseases: Springer.
Manohara, D.,Wahyuno, D., Mulya, K., Sutrasman dan Sudradjat, A., 2004. Pengendalian patogen penyakit busuk pangkal batang lada dengan cara pengelolaan tanaman. Laporan Hasil Penelitian Proyek PHT Perkebunan Rakyat. Balai Penelitian Tanaman Rempah dan Obat.
Mansfied, J., Genin, S. Magor, S., et al. 2012. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 13:614–629.
Maryani, N., Lombard, L., Poerba, Y.S., Subandiyah, S., Crous, P.W., and Kema, G.H.J. 2018. Phylogeny and genetic diversity of the banana Fusarium wilt pathogen Fusarium oxysporum f. sp. cubense in the Indonesian centre of origin. Studies In Mycology 92: 155–194.
Mihajlović. M., Rekanović, E., Hrustić, J., Grahovac, M., and Tanović, B. 2017. Methods for management of soilborne plant pathogens. Pestic. Phytomed. (Belgrade), 32(1), 2017, 9–24.
Milus, E.A. & Parsons, C.E. 1994. Evaluation of foliar fungicides for controlling Fusarium head blight of wheat. Plant Dis. 78, 697-699.
Montesinos, E. 2003. Development, registration and commercialization of microbial pesticides for plant protection. Int Microbiol (2003) 6: 245–252 DOI 10.1007/s10123-003-0144-x.
Mutala’liah, Indarti S , Wibowo A. 2019. Short Communication: The prevalence and species of root-knot nematode which infect on potato seed in Central Java, Indonesia. Biodiversitas 20: 11-16.
Nasahi, C., Widiantini, F., Yulia, E., Meliansyah, R., Rasisetyo, P. 2016. Isolasi dan deteksi potensi aktinobacteria endofit dalam mengendalikan penyakit busuk pangkal batang pada tanaman kelapa sawit (Ganoderma boninense Pat.). Di Dalam: Joko T, editor. Prosiding Seminar Nasional Pengendalian Penyakit pada Tanaman Ramah Lingkungan II, 2016 Agustus 27. Yogyakarta (ID): Perhimpunan Fitopatologi Indonesia. hlm. 66–78.
Nasir, N., Pittaway, P.A., Pegg, K.G., et al. 1999. A pilot study investigating the complexity of Fusarium wilt of bananas in West Sumatra, Indonesia. Australian Journal of Agricultural Research 50: 1279–1283.
Nelson, G.A. 1982. Corynebacterium sepedonicum in potato: effect of inoculum concentration on ring rot symptoms and latent infection. Can J Plant Pathol 4:129–133.
Nion, Y.A., and Toyota, K. 2008. Suppression of bacterial wilt and fusarium wilt by Burkholderia nodosa strain isolated from Kalimantan soils, Indonesia. Microbes Environ. Vol. 23, No.2, 134-147.
Noble, R., E. Coventry. 2005: Suppression of soil-borne plant diseases with composts: a review. Biocontrol Sci. Technol. 15, 3-20.
Nugrahaeni, N. 2011. Pemuliaan kacang tanah untuk ketahanan terhadap layu bakteri Ralstonia di Indonesia. Bul. Palawija. No. 21:1–12.
Paterson, R.R.M. 2019. Ganoderma boninense Disease of Oil Palm to Significantly Reduce Production After 2050 in Sumatra if Projected Climate Change Occurs. Microorganisms, 7, 24; doi:10.3390/microorganisms7010024.
Pedai, T., Hadisutrisno, B., Priyatmojo, A. 2015. Utilization of Arbuscular Micorrhizal Fungi To Control Fusarium Wilt Of Tomatoes. Jurnal Perlindungan Tanaman Indonesia, Vol. 19, No. 2, 2015: 89–93.
Pimentel, D., Cooperstein, S., Randell, H., Filiberto, D., Sorrentino, S., Kaye, B., Nicklin, C., Yagi, J., Brian, J., & O’Hern, J., Habas, A., Weinstein, C. 2007. Ecology of Increasing Diseases: Population Growth and Environmental Degradation. Hum. Ecol. 35:653–668 DOI 10.1007/s10745-007-9128-3.
Postma, J., M. Montanari, M, Van Den Boogert, P.H.J.F. 2003: Microbial enrichment to enhance the disease suppressive activity of compost. Eur. J. Soil Biol. 39, 157-163.
Price, T.V. 2000. Plant parasitic nematodes. Prosiding pelatihan nematologi. Jakarta. 16-30 Juli 2000. Pusat Karantina Pertanian. Jakarta. Hal 27-34.
Prihatna, C., Barbetti, M.J., and Barker, S.J. 2018. A novel tomato Fusarium wilt tolerance gene. Front. Microbiol. 9: 1226. doi: 10.3389/fmicb.2018.01226.
Pugliese M, Benetti A, Gilardi G, Gullino ML, Garibaldi A. 2014. Control Of Soil-Borne Diseases by Different Composts In Potted Vegetable Crops. Commun Agric Appl Biol Sci. 2014;79(2): 37-40.
Rees, R.W., Flood, J., Hasan, Y., Wills, M.A., and Cooper, R.M. 2012. Ganoderma boninense basidiospores in oil palm plantations: evaluation of their possible role in stem rots of Elaeis guineensis. Plant Pathology 61, 567–578.
Rejeki Siti Ferniah, Rina Sri Kasiamdari, Achmadi Priyatmojo and Budi Setiadi Daryono. (2018). Resistance response of chilli (Capsicum annuum L.) F1 to Fusarium oxysporum involves expression of the CaChi2 gene. Tropical Life Sciences Research 29(2): 29–37. https://doi.org/10.21315/tlsr2018.29.2.3.
Riska, J., Hermanto, C. 2012. Hubungan antara tingkat konsentrasi inokulum Fusarium oxysporum f. sp. cubense VCG 01213/16 dengan 77 perkembangan penyakit layu pada kultivar pisang rentan. J Hort 22(2): 156-164.
Ryckeboer, J., Mergaert, J., Vaes, K., Klammer, S., De Clercq, D., Coosemans, J., Insam, H., Swings, J. 2003. A survey of bacteria and fungi occurring during composting and self-heating processes. Annals of Microbiology, 53 (4), 349-410.
Safitri, D., Soekarno, B.P.W., Achmad, Surono. 2012. Pemanfaatan bakteri endofit untuk meningkatkan ketahanan lada terhadap serangan Phytopthora capsici Leon penyebab penyakit busuk pangkal batang (Utilization of endophytic bacteria to increase pepper plant resistance to attack of Phytopthora capsici Leon causes stem rot disease). Proceeding of Seminar Nasional Mikologi, Universitas Jenderal Soedirman, Indonesia (National Seminar on Mycology, Jenderal Soedirman University, Indonesia), p. 489-499.
Sakpetch, P., H-Kittikun, A., Kuwahara, Y., Komeda, H., Asano, Y., 2018. Isolation of indigenous antagonistic microorganism to inhibit Rigidoporus microporus and other plant pathogens and analysis of the bioactive compounds. Biological Control, 124, 53–60.doi:10.1016/j.biocontrol.2018.01.007.
Schell MA. 2000. Control of virulence and pathogenicity genes of Ralstonia solanacearum by an elaborate sensory network. Annu Rev Phytopathol 38: 263-292.
Setyorini, D., R. Saraswati, dan E.K. Anwar. 2006. Kompos. P: 11 – 40. In R.D.M. Simanungkalit, D.A. Suriadikarta, R. Saraswati, D. Setyorini, dan W. Hartatik. Pupuk organic dan pupuk hayati. Balai Besar Litbang Sumberdaya Lahan Pertanian.
Simanungkalit, R.D.M., Ardi, D., Saraswati, R., Setyorini, D., Hartatik, W. 2006. Pupuk Organik dan Pupuk Hayati. Balai Besar Penelitian dan Pengembangan Sumberdaya Lahan Pertanian, Bogor. 14p.
Soekarno, B.P.W., Surono, Hendra. 2013. Optimalisasi peran kompos bioaktif dengan penambahan asam humat dan asam fulvat untuk meningkatkan ketahanan tanaman mentimun terhadap serangan Pythium sp. (Optimizing the role of bioactive compost with the addition of humic acid and fulvic acid to increase the cucumber plant resistance against Pythium sp.). Bionatura-Jurnal Ilmu-ilmu Hayati dan Fisik, Vol. 15, No. 1, March 2013: 35 – 43.
Soekarno, B.P.W., Surono, Rahmania, A. 2012. Keefektifan asam humat dan bakteri aktivator pada kompos untuk pengendalian rebah kecambah oleh Schlerotium rolfsii Sacc. pada Kacang Tanah (The effectiveness of humic acid and activator bacteria in the compost to control damping off disease caused by Schlerotium rolfsii Sacc. in peanut plant). Proceeding of Seminar Nasional Mikologi, Universitas Jenderal Soedirman, Indonesia (National Seminar on Mycology, Jenderal Soedirman University, Indonesia), p. 472-481.
Soekarno, B.P.W., Surono, Susanti. 2014. Formulasi Pelet Berbahan Aktif Trichoderma sp. untuk Pengendalian Penyakit Rebah Kecambah (Pythium sp.) pada Tanaman Mentimun (Pellet Formulation with Active Material of Trichoderma sp. for Controlling Damping Off Disease in Cucumber Plants). Jurnal Fitopatologi Indonesia (Indonesian Journal of Phytopathology) : Volume 10, Nomor 5, p 153–159, DOI: 10.14692/jfi.10.5.153.
Subowo, Purwani, J., and Rochayati, S. 2014. Biofertilizer Development Prospects and Challenges for Improved Soil Fertility. Jurnal Sumberdaya Lahan Vol 7 No. 1: 15-26.
Supyani and Widadi, S.. 2015. Hypovirulent Isolates of Fusarium Collected From Chili Crops in Boyolali Regency, Central Java, Indonesia. Agrivita Volume 37 No. 1: 67-74. http://dx.doi.org/10.17503/Agrivita-2015-37-1-p067-074.
Surono and Narisawa, K. (2017). The dark septate endophytic fungus Phialocephala fortinii is a potential decomposer of soil organic compounds and a promoter of Asparagus officinalis growth. Fungal Ecol. 28, 1–10. doi: 10.1016/j.funeco.2017.04.001.
Surono and Narisawa, K. (2018). The inhibitory role of dark septate endophytic fungus Phialocephala fortinii against Fusarium disease on the Asparagus officinalis growth in organic source conditions. Biological Control 121: 159-167. https://doi.org/10.1016/j.biocontrol.2018.02.017.
Surono, Soekarno, B.P.W., Alfian Muri L. 2012. Controlling Fusarium damping of on tomato using bioactive compound enriched by activator microbe and humic acid. As. J. Food Ag-Ind., 5(06), 476-484.
Surono. 2017. The role of dark septate endophytic fungus, Phialocephala fortinii, on promoting Asparagus officinalis growth under various stressed conditions. Doctoral Thesis, Tokyo University of Agriculture and Technology, Japan.
Surono. 2018. Isolation and selection of dark septate endophytic fungi (DSE) to promote rice growth in acidic and blast disease (Pyricularia oryzae) stress conditions. Final Report of SMARD Research Grant, the Indonesian Agency for Agricultural Research and Development (IAARD), the Ministry of Agriculture, INDONESIA, pp 71.
Syafi'i, D.D., Lisnawita, Hasanudin. 2018. Distribution of Potato Cyst Nematode in Wonosobo and Banjarnegara, Central Java. Volume 14, Nomor 4, Juli 2018 Halaman 111–119 DOI: 10.14692/jfi.14.4.111.
Tiquia, S.M., Michel, F.CJr. 2002. Bacterial Diversity in Livestock manure compost as characterized by terminal restriction fragment length polymorphisms (T-RFLP) of PCRamplified 16S rRNA gene sequences.
Widiantini, F., Nasahi, C., Yulia, E., Noviyawati, S. 2018. Potency of Endophytic Bacterial Secondary Metabolite to Inhibit Mycelium Growth of Ganoderma boninense. Volume 14, Nomor 3, Mei 2018 Halaman 104–109 DOI: 10.14692/jfi.14.3.104.
Yulianti, Nidar, T. 1999. Pertanian organik dan penyakit tanaman. Prosiding: Kongres Nasional XV dan Seminar Ilmiah Perhimpunan Fitopatologi Indonesia. Universitas Jenderal SUdirman. Purwokerto, 2000. Hal.: 592.
Yuliar, Nion, Y.A., and Toyota, K. 2015. Recent Trends in Control Methods for Bacterial Wilt Diseases Caused by Ralstonia solanacearum. Microbes Environ. Vol. 30, No. 1, 1-11.
Zaffan, Z.R., Soekarno, B.P.W., Munif, A., and Surono. 2018. Potential of Indonesia’s Indigenous Dark Septate Endophytic Fungi To Control Fusarium Wilt In Vitro. 3nd International Conference on Tropical Biology Ecological Restoration in Southeast Asia: Conservation, Enhancement and Sustainable Use of Indigenous Tropical Flora and Fauna. SEAMEO BIOTROP, Bogor-Indonesia, 20-21 September 2018.


The Soil-Borne Pathogen/Disease Problems and Management Strategies: The Indonesia Experience
ABSTRACT
Soil-borne diseases are one of the factors that limit the productivity of agricultural crops in Indonesia. Soil-borne pathogens that predominantly attack various types of agricultural plants in Indonesia include Fusarium spp., Pythium spp., Phytophthora spp., Ralstonia solanacearum, Ganoderma boninense, and Rigidoporus microporus. Sometimes the losses caused by the attack of soil-borne pathogens are very high in economic terms. Management of controlling soil-borne diseases in Indonesia is by using organic fertilizers, both those which are enriched with beneficial microbes and minerals or not, the use of biological fertilizers including biopesticides, and improvement of cultivation systems that suppress the development of the soil-borne diseases. One potential that is being developed is research and development of endophyte dark septate fungi (DSE)-based on fertilizer technology to promote plant growth and production under biotic and abiotic stress conditions including the control of soil-borne diseases in Indonesia.
Keywords: Organic fertilizer, Bio-fertilizer, Bio-pestices, Soil-borne diseases, Dark septate endophytic fungi
INTRODUCTION
Fertile soil does not always indicate that the soil is healthy. The abundant pathogenic microbial population in that soil may cause a decline in the crop productivity although the soil is abundant with rich nutrients (Fiers et al., 2012; Frac et al., 2018). Soil-borne diseases are more restrictive in the production of many agricultural crops and contribute 10-20% of yield losses annually compared with seed-borne and air-borne diseases (Yuliar et al., 2015). For example, the resulting losses due to soil borne diseases caused by nematodes in plants around the world are estimated at US$ 80 billion per year (Price, 2000). The distribution of nematode attacks is not only in vegetables but also includes food crops and plantations such as Meloidogyne incognita, M. javanica, and M. arenaria which is the most destructive root-knot nematode to infect potato in Indonesia (Mutala’liah et al., 2018). Syafi'i et al. (2018) stated that the potato cyst nematode, Globodera rostochiensis, is increasingly widespread in potato plantations in Wonosobo and Banjarnegara, Central Java, Indonesia, and if not controlled it will extend to other parts of Indonesia.
Soil-borne pathogen bacteria, Ralstonia solanacearum, that causes bacterial wilt, has an extensively wide host, over 200 species (Schell, 2000; Yuliar et al., 2015) and still a major obstacle in many crop cultivation in Indonesia and induces a destructive economic impact (Kelman, 1998; Yuliar et al., 2015). This disease is included in the five main diseases in several countries and causes considerable loss of yield (20-45%), the quality of the seed produced is low and causes land contamination so that it cannot be planted for a long time (Mansfied et al., 2012; Yuliar et al., 2015). In Indonesia, R. solanacearum is the main factor that causes low yields of peanuts (Nugrahaeni, 2011). R. solanacearum attacks economically important plants, such as tomatoes, potatoes, tobacco, bananas, and peanuts (Hayward 1991; Elphinstone 2005; Hemelda et al., 2019). R. solanacearum has become an important pathogen in agriculture in Java, Indonesia (Hemelda et al., 2019).
Fusarium oxysporum is an important pathogen that attacks various economically valuable plants in Indonesia such as tomatoes, chilies, shallot, onion, and banana (Nasir et al., 1999; Nion and Toyota, 2008; Hwang and Ko, 2014; Supyani and Widadi, 2015; Pedai et al., 2015; Prihatna et al., 2018; Maryani et al., 2019). This pathogen can attack its host plants in all phases of growth (Cahyono, 2008; Mihajlović et al., 2017). In tomato plants, losses caused by the attack of these pathogens in Indonesia can reach 20-30% (Wibowo, 2007). Fusarium wilt caused by F. oxysporum is also a potential pathogen of chilies in Indonesia (Ali, 2006; Ferniah et al., 2018). Nasir et al. (1999) reported that Fusarium wilt on banana plants occurs in almost all parts of Indonesia from Aceh to Papua (Maryani et al., 2019). The impact of the Fusarium oxysporum attack on banana plants is the destruction of thousands of hectares of banana plantations, both commercial banana plantations and people's banana plantations (Riska and Hermanto, 2012).
Soil-borne diseases also attack the main crops of plantations in Indonesia such as rubber, oil palm and pepper. Soil-borne pathogen, Ridigoporus microporus attacks rubber plants in all phases of growth and causes huge economic losses in Indonesia (Kaewchai and Soytong, 2010; Sakpetch et al., 2018). Whereas in oil palm plants, a large economic loss is caused by the attack of soil-borne pathogen, Ganoderma boninense, which attacks both in the nursery phase and the plant phase to produce fruit and as a most serious disease in oil palm plantation in Indonesia (Darmono et al., 2000; Rees et al., 2012; Lisnawati et al., 2016; Paterson, 2019). In pepper plants, Phytophthora capsici, is a soil-borne pathogen that attacks pepper plantations in Lampung, Indonesia and gives great damage to people's pepper plantations (Manohara et al., 2004; Hendra et al., 2014).
Table 1. Some soil-borne pathogens and their host plant distribution and the diseases caused by these pathogens in Indonesia
No
Soil-borne pathogen
Host plants
Disease
Fungi
1
Fusarium spp.
Tomato, banan, shallot, chilli, melon, legume crops
Fusarium wilt, damping off
2
Pythium spp.
Cucumber
Damping off
3
Phythopthora spp.
Pepper, orange, cacao, tobacco, vegetables, legume crops
Phytophthora foot rot, blight and fruit rot
4
Ganoderma boninense
Oil palm
Basal stem rot
5
Rigidoporus microporus
Rubber
White root rot
Bakteri
1
Ralstonia solanacearum
Tomato, banana, potato, ginger, peanut
Bacterial wilt
Nematode
Food crops, horticultural crops and plantation crop
Root galls
Processed from various sources
Soil health in terms of soil biological aspects
The quality of soil biology in an agricultural land resource is an important component and one of the factors that determine the sustainability of the function of the land to support optimal and sustainable soil and plant productivity (Abawi and Widmer, 2000; Frac et al., 2018). The “sick soil” will cause plants that grow on that soil to be sick too, which will significantly decline plant productivity (Huang et al., 2006). The health aspects of the soil related to soil biological conditions is an important component that is often forgotten in determining the quality of agricultural land (Abawi and Widmer, 2000). The term sick soil is not only limited to marginal soils that are infertile due to the unavailability or lack of nutrients available that can be used by plants but also includes soils which have an abundance of soil pathogen population or severe outbreaks of soil-borne diseases (Huang et al., 2006). Texture, pH, organic matter, temperature and soil nutrients strongly influence the activity of soil-borne pathogens and soil health (Fiers et al., 2012).
Soil health refers to the capacity of the soil to maintain environmental quality, sustain biological productivity, and promote plant health (Frac et al., 2018; Haney et al., 2018). Current soil and plant health issues are no longer just concerns of plant disease scientists but become also the concern of soil scientists to develop sustainable soil health management which combines improved environmental quality, biological productivity and plant health (Frac et al., 2018). Soil-borne diseases have become one of the limiting factors for productivity in various types of plants, both horticulture, food and plantations (Mihajlovic et al., 2017; Katan, 2017). Soil-borne diseases can be latent because some pathogens that cause infectious diseases can live decades in the soil to interact again with host plants and survive in the soil from season to season (Nelson, 1982; Lucas, 2006; Fiers et al., 2012; Lecreck et al., 2014).
Recently, the control practices of soil-borne diseases in Indonesia use chemical pesticides such as fungicides and bactericides on a large scale. Under certain conditions or short-term, the use of pesticides will eradicate the target pathogen with great satisfaction to farmers, but useful microbes will also be eradicated so that the balance of the microflora and soil fauna is disrupted, in the long run there is a very severe attack and the emergence of resistant pathogens (Montesinos, 2003; Pimentel et al., 2007; Aktar et al., 2009). In addition, the use of pesticides causes soil pollution and the condition of the soil is getting sicker (Pimentel et al., 2007; Aktar et al., 2009). The problem of pest and plant disease resistance can occur due to improper use of pesticide compounds (Milus and Parsons, 1994). Therefore, technology is now being sought to improve soil health and control soil-borne diseases by using environmentally friendly active ingredients such as organic fertilizers (compost), biological fertilizers and biopesticides for the control of major soil-borne diseases in Indonesia based on conventional and molecular approaches.
Soil-borne disease management strategies: promoting the role of organic and biological fertilizers (plant growth promoter and bio-control/biopesticide) in improving soil health and controlling soil-borne diseases in Indonesia
Organic fertilizers such as compost is a complete source of macro and micro nutrients even in low quantities and can be used by plants to grow and produce (Setyorini et al., 2006) so that high yields of compost doses are needed to obtain adequate results. The presence of beneficial microbial community in compost can suppress pathogenic populations in the soil (Soekarno et al., 2013; Pugliese et al., 2014). Some microbial groups of antagonist bacteria (Bacillus spp., Pseudomonas spp.) and fungi (Trichoderma spp., Aspergillus spp., Penicillium spp.) are the most microbes found in compost (Tiquia and Michel, 2002; Ryckeboer et al., 2003). One of the environmentally friendly control strategies for soil-borne disease in vegetable crops is to use compost or other organic amendment, which results depend on the type of compost, application level and pathosystem (Bailey and Lazarovits, 2003; Pugliese et al., 2014).
The application of compost to increase organic carbon content in the soil will also increase beneficial soil microbial populations that suppress soil-borne pathogen (Noble and Coventry, 2005). Compost will stimulate soil microbial activity because it provides a substrate for microbes to active and become more dominant than pathogenic microbes (Pugliese et al., 2008). The addition of compost can increase the growth of soil microbes that are antagonistic to pathogens (Yulianti and Nidar, 1999). The results of the study of Carrise et al (2003) and Postma et al. (2003) showed that microbes from compost are able to suppress Pythium ultimum in cucumber and Verticillium biguttatum in sugar beets and tomatoes. The results of the research conducted by Soekarno et al. (2013) showed that the addition of compost enriched with PGPR bacteria (SR1L4 and SR2C3R1 bacteria), humic acid, and fulvic acid was able to increase the diversity of soil microbial populations in cucumber plant growth media and suppress Pythium sp attacks, causing damping off disease.
Table 2. Effect of growing media content on the diversity of soil microbial populations in cucumber plant growing media
Soil phase contions
Growing media content
Bacteria (cfu/ml)
Fungi (cfu/ml)
Actinomycetes (cfu/ml)
Before treatments
X
3.50.107
2.00.103 – 1.50.105
0
Z
3.00.105 – 2.50.107
2.00.103
0
After treatments
X
1.35.106 – 3.30.108
1.50.105
0
Y
2.75.106 – 3.50.108
0.50.105 – 0.50.107
0
Z
10.9.106 – 28.1.108
2.00.105
1.50.105
AH
24.8.106 - 5,50.108
30.105 – 2.50.107
4.50.105 - 2.00.107
AF
19.0.106 – 26.3.108
9.00.105 - 8.00.107
2.00.105 - 1.00.107
AHF
10.3.106- 2.50.108
6.50.105 - 4.00.107
1.00.107
SR1
29.6.106 – 7.05.108
29.5.105 – 4.50.107
3.50.105 – 0.50.107
SR2
18.2.106- 1.05.108
14.0.105 – 2.50.107
7.50.105 - 2.00.107
SR3
11.3.106 – 1.30.108
15.0.105 – 8.50.107
1.00.105 – 1.50.107
Note :
x: soil without compost, humic acid, fulvic acid, bacterium (negative control), y: sterilized soil without compost, humic acid, fulvic acid, bacterium (positive control), z: compost treatment AH : compost+humic acid, AF : compost+fulvic acid, AHF : compost+humic acid+fulvic acid,
SR1 : compost+bacterium SR1L4, SR2 : compost+bacterium isolate SR2C3R1, SR3 : compost+bacterium isolate SR1L4+bacterium isolate SR2C3R1
(Source : Soekarno et al., 2013)
Addition of organic matter especially compost can increase soil microbial activity because more energy sources are provided and soil conditions are made better for soil microbial activity and development (Bailey and Lazarovits, 2003; Pugliese et al., 2014). According to Campbell (1989) the use of organic fertilizers in addition to improving soil structure and providing nutrients, suppresses germination of pathogenic spores, causes lysis of pathogenic microbial cells, deactivates transient and permanent pathogen growth, supports non-activity -pathogen in providing nutrients and growth stimulating compounds for plants so as to improve the health of soil and plants. Surono et al. (2012) stated that compost enriched by humic acid and activator bacterium BX2 gives a positive effect on inhibiting infection of Fusarium sp. causing damping off in tomato plants and improves the agronomic potential of tomato plants. Based on research by Hendra et al. (2014) the use of coffee skin compost enriched with zeolite, humic acid, fulvic acid and microbial activator can suppress the attack of soil-borne pathogens Phythopthora capsici which causes rot of the stem at around 50% pepper.
In addition to organic fertilizers, there is another type of fertilizer that has a role in promoting plant growth and suppressing plant disease attacks, namely biofertilizer. Biofertilizer contains beneficial live organisms that function to facilitate the availability of nutrients in the soil for plants (Simanungkalit et al., 2006), to promote plant growth and to control plant disease (Surono and Narisawa, 2018). In addition to pathogenic microbes, in the soil, there are also various types of microbes that are beneficial for soil health. These microbes consist of bacteria, actinomycetes, fungi, and non-pathogenic nematodes. These beneficial soil microbes develop according to their soil habitat conditions. The functions of these soil microbes include nitrogen fixation, phosphate solubilizer, production of growth regulators and controlling diseases including soil-borne disease (Baum et al., 2015; El-Komy et al., 2015; Frac et al., 2018). Various beneficial soil microbes have been developed into biological fertilizer products that serve to support soil and plant productivity.
Formulations of various types of microbes to be used as biological fertilizer formulas are now rapidly being developed especially in Indonesia. Various products have been in the market with various kinds of active microbial contents. Generally, biofertilizer products developed in Indonesia are biofertilizers containing nitrogen-fixing bacteria, phosphate solubilizer, plant growth promoter and plant disease controllers, including soil-borne disease controller (Direktorat Pupuk dan Pestisida, 2015). For the most part, biofertilizer products are made from microbial active substances such as Bacillus, Pseudomonas, and Trichoderma (Direktorat Pupuk dan Pestisida, 2015).
Soekarno et al. (2014) stated that the use of Trichoderma active pellet formula is very effective in suppressing the growth of Pythium sp. causes of damping off disease. Surono et al. (2012) stated that the application of compost-enriched by bacterium BX2 and humic acid increased the population of soil microbes in growth media of tomato plants thus enhanced both soil and plant health. Based on the results of research by Soekarno et al. (2012) that selected bacterium B6 isolate-enriched compost applied to soil as a growing medium for peanut plants infested with Sclerotium rolfsii was able to control the incidence of damping off disease caused by that soil-borne pathogen in peanut plants by 93.75%. Safitri et al. (2012) stated that the application of endophytic bacteria was able to suppress Phytophthora foot rot disease from 51.95% to 95.63% in black pepper seedlings and the disease severity in ranged of 1,28%-29,35%. Endophytic bacteria isolated from the roots of oil palm plants can suppress the growth of G. boninense (Nasahi et al., 2016) and the compounds produced by endophytic bacteria are also able to inhibit the growth of G. boninense (Widiantini et al., 2018). Development of biological fertilizers should consider site-specific conditions with indigenous microbial resources that have been available in the field because the conditions of agricultural land in Indonesia have high diversity or heterogeneity (Subowo et al., 2013).
Research and development of dark septate endophytic fungi (DSE)-based fertilizer and pesticide for major soil-borne disease control in Indonesia
Surono and the team from the Indonesian Soil Research Institute (ISRI) and Bogor Agricultural University (IPB University) have researched and developed DSE isolated from various agroecosystems in Indonesia. DSE fungi have been isolated and selected both in laboratories, greenhouses and fields. Various types of DSE have been collected and examined for their benefits to stimulate the growth of various plants under biotic and abiotic stress conditions including for the control of soil-borne diseases such as Fusarium oxysporum f.sp. lycopersici in tomatoes (Zuhay et al., 2018), can suppress the development and attack of Ridigodoporus microporus in rubber plants (Surono et al, 2019, not yet published) both in the laboratory and in rubber plant nurseries and can suppress the growth of Ganoderma boninense in oil palm plants ( Surono et al, not yet published). The research conducted by Surono and Narisawa (2018) states that the endophyte dark septate fungus, Phialocephala fortinii, is capable of suppressing Fusarium oxysporum f.sp. asparagi on asparagus plants and increase the growth of asparagus in organic conditions (Surono and Narisawa, 2017). Not only suppresses soil-borne disease, DSE isolated by Surono et al. (2018) able to suppress blast disease caused by Phyricularia oryzae in rice plants. Research related to DSE fungal based on conventional and molecular approaches is underway.
Indonesian programs and achievements on the use of organic and biological fertilizers to improve soil and plant health in Indonesia
Soil-borne diseases are increasing everywhere in Indonesia, especially in the face of climate change, which now often occurs in an extreme way which affects the production of agricultural products, although the attack of the soil-borne disease still gets less attention compared to air-borne and seed-borne disease in the field (Yuliar et al., 2015; Syafi’i et al., 2018; Mutala’liah et al., 2018; Maryani et al., 2018; Hemelda et al., 2019). This often encourages farmers to use pesticides excessively without producing the dosage that should be given.
Based on the results of several studies that have been conducted (Surono et al., 2012; Soekarno et al., 2013; Soekarno et al., 2012; Safitri et al., 2012; Hendra et al., 2014), organic fertilizers, especially compost in addition to being used as a plant nutritional sources, can also be used to control soil-borne diseases such as damping off, basal stem rot, foot rot, and white root rot caused by soil-borne pathogens. Therefore, the use of organic fertilizers needs to be encouraged and increased in the use in the field by Indonesian farmers and agro-industry sectors. In addition to organic fertilizers, biological fertilizers which contain active ingredients are beneficial microbes especially use indigenous Indonesian microbes that can be synergistically with organic fertilizer utilized to support sustainable crop production. The Indonesian government has supported this program by implementing an organic fertilizer subsidy program and direct assistance to farmers in the form of organic and biological fertilizers, and equipment and machinery to support organic fertilizer production directly by farmers.
The use of organic and biological fertilizers can have an impact on environmental health. Various studies in the laboratories, greenhouses, and fields have been carried out by showing results that satisfy the positive influence in controlling the soil-borne disease by using organic and biological fertilizers. These potentials and opportunities require policy direction from the Government so that relevant stakeholders can develop technology in a targeted manner based on existing protocols so as not to harm the community, especially farmers as end users in the field. Through the Research Center and in synergy with the Observation Centers for Plant Pest and Disease Organisms under the Ministry of Agriculture and Local Government, environmentally friendly technologies for controlling soil-borne disease and other diseases are disseminated to Indonesian farmers through counseling, extension, and integrated agricultural pest and disease control schools.
CONCLUSION
Indonesian farmers' knowledge of benefits, how to use, and how to obtain organic fertilizers and biological fertilizers increase by training and field farmer school. Some farmer communities have practiced producing biofertilizers what they call local microorganisms (MOL), which are biological fertilizers that contain various types of microbes, in the cultivation of their plants even without clear quality control. Research on organic and biological fertilizers to control soil-borne pathogens both in the laboratory, greenhouse and field fields has shown satisfactory results. To speed up the process of spreading the use of organic and biological fertilizers without or combined between them to suppress soil-borne diseases, it needs to be developed, promoted and promoted through extension activities in the form of demonstration plots and socialization and training, workshops and seminars. The Indonesian Agency for Agricultural Research and Development (IAARD) in collaboration with the Local Government of Agriculture Office supported by the private sector increasingly improve demonstration plots, leaflets, booklets, and training as an active means of disseminating this program.
REFERENCES
Abawi, G.S., Widmer, T.L. 2000. Impact of soil health management practices on soilborne pathogens, nematodes and root diseases of vegetable crops. Applied Soil Ecology 15: 37–47.
Aktar, M.W., Sengupta, D., Chowdhury, A. 2009. Impact of pesticides use in agriculture: their benefits and hazards. Interdisc Toxicol. Vol. 2(1): 1–12. doi: 10.2478/v10102-009-0001-7.
Ali, M. 2006. Chili (Capsicum spp.) food chain analysis: Setting research priorities in Asia. Technical Bulletin No. 38. AVRDC - The World Vegetable Center, Taiwan.
Bailey, K.L., Lazarovits, G.. 2003. Suppressing soil-borne diseases with residue management and organic amendments. Soil & Tillage Research 72: 169–180.
Baum, C., El-Tohamy, W., and Gruda, N. 2015. Increasing the productivity and product quality of vegetable crops using arbuscular mycorrhizal fungi: a review. Sci. Hortic. 187, 131–141. doi: 10.1016/j.scienta.2015.03.002.
Cahyono, B. 2008. Tomat Usaha Tani dan Penanganan Pasca Panen. Kanisius: Yogyakarta.
Campbell, R., 1989. Biological Control of Microbial Plant Pathogens. 1st Edn., Cambridge University Press, Cambridge, ISBN: 0 521 34900 1.
Carisse, O., Bernier, J., Benhamou, N. 2003: Selection of biological agents from composts for control of damping-off of cucumber caused by Pythium ultimum. Plant Pathol. 25, 258-267.
Darmono, T. 2000. Ganoderma in oil palm in Indonesia: Current status and prospective use of antibodies for the detection of infection. In Ganoderma Diseases of Perennial Crops; Flood, J., Bridge, P.D., Holderness, M., Eds.; CABI Publishing: Wallingford, UK; pp. 249–266.
Direktorat Pupuk dan Pestisida, Direktorat Jenderal Prasarana dan Sarana Pertanian, Kementerian Pertanian Indonesia. 2015. Pupuk Terdaftar.
El-Komy, M. H., Saleh, A. A., Eranthodi, A., and Molan, Y. Y. 2015. Characterization of novel Trichoderma asperellum isolates to select effective biocontrol agents against tomato Fusarium wilt. Plant Pathol. J. 31, 50–60. doi: 10.5423/PPJ.OA.09.2014.0087.
Elphinstone JG. 2005. The current bacterial wilt situation: A global view. In: Allen C, Prior P, Hayward AC (eds) Bacterial Wilt Disease and the Ralstonia solanacearum Species Complex. American Phytopathological Society Press, Minnesota.
Fiers, Edel-Hermann, Chatot, Hingrat, Alabouvette, et al. 2012. Potato soil-borne diseases. A review. Agronomy for Sustainable Development, Springer Verlag/EDP Sciences/INRA, 32 (1), pp.93- 132. ff10.1007/s13593-011-0035-zff. ffhal-00930506.
Frac, M., Hannula, S.E., Bełka, M. and Jedryczka, M. 2018. Fungal Biodiversity and Their Role in Soil Health. Front. Microbiol. 9:707. doi: 10.3389/fmicb.2018.00707.
Haney, R.L., Haney, E.B., Smitha, D.R., Harmela, R.D., White, M.J. 2018. The soil health tool—Theory and initial broad-scale application. Applied Soil Ecology 125: 162–168.
Hayward, A.C. 1991. Biology and epidemiology of bacterial wilt caused by P. solanacearum. Ann. Rev. Phytopath. 29:67–87.
Hemelda NM, Safitri R, Suhandono S. 2019. Genetic diversity of Ralstonia solanacearum, A phytopathogenic bacterium infecting horticultural plants in Java, Indonesia. Biodiversitas 20: 364-372.
Hendra, J., Widodo, Soekarno, B.P.W., Bintoro, H.M., Manohara, D. 2014. Perspektif Pengembangan Kompos Bioaktivator untuk Pengendalian Phytophthora capsici pada Tanaman Lada (Piper nigrum). Jurnal Pengkajian dan Pengembangan Teknologi Pertanian Vol. 17, No.1,: 37-48.
Huang, H.C., Chou, C.H., Erickson, R.S. 2006. Soil sickness and its control. Allelopathy Journal 18(1): 1-21.
Hwang, S.C., Ko, W.H. 2014. Cavendish banana cultivars resistant to fusarium wilt acquired through somaclonal variation in Taiwan. Plant Dis. 88(6): 580-588. doi:10.1094/PDIS.2004.88.6.580.
Kaewchai, S., Soytong, K., 2010. Application of biofungicides against Rigidoporus microporus causing white root disease of rubber trees. Journal of Agricultural Technology 2010. 6(2), 349-363.
Katan, J. 2017. Diseases caused by soilborne pathogens: biology, management and challenges. Journal of Plant Pathology, 99 (2): 305-315.
Kelman, A. 1998. One hundred and one years of research on bacterial wilt, p. 1–5. In P.H. Prior, C. Allen and J. Elphinstone (ed.), Bacterial Wilt Disease: Molecular and Ecological Aspects. Springer, Heidelberg.
Leclerc, M, Dore´, T., Gilligan, C.A,. Lucas, P., Filipe, J.A.N. 2014. Estimating the Delay between Host Infection and Disease (Incubation Period) and Assessing Its Significance to the Epidemiology of Plant Diseases. PLoS ONE 9(1): e86568. doi:10.1371/journal.pone.0086568.
Lisnawita, Hanum, H, Tantawi, A.R. 2016. Survey of Basal Stem Rot Disease on Oil Palms (Elaeis guineensis Jacq.) in Kebun Bukit Kijang, North Sumatera, Indonesia. International Conference on Agricultural and Biological Sciences (ABS 2016), Earth and Environmental Science. 2016; (41)012007.
Lucas, P. 2006. Diseases caused by soil-borne pathogens. In: Cooke BM, Jones DG, Kaye B, editors. The Epidemiology of Plant Diseases: Springer.
Manohara, D.,Wahyuno, D., Mulya, K., Sutrasman dan Sudradjat, A., 2004. Pengendalian patogen penyakit busuk pangkal batang lada dengan cara pengelolaan tanaman. Laporan Hasil Penelitian Proyek PHT Perkebunan Rakyat. Balai Penelitian Tanaman Rempah dan Obat.
Mansfied, J., Genin, S. Magor, S., et al. 2012. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 13:614–629.
Maryani, N., Lombard, L., Poerba, Y.S., Subandiyah, S., Crous, P.W., and Kema, G.H.J. 2018. Phylogeny and genetic diversity of the banana Fusarium wilt pathogen Fusarium oxysporum f. sp. cubense in the Indonesian centre of origin. Studies In Mycology 92: 155–194.
Mihajlović. M., Rekanović, E., Hrustić, J., Grahovac, M., and Tanović, B. 2017. Methods for management of soilborne plant pathogens. Pestic. Phytomed. (Belgrade), 32(1), 2017, 9–24.
Milus, E.A. & Parsons, C.E. 1994. Evaluation of foliar fungicides for controlling Fusarium head blight of wheat. Plant Dis. 78, 697-699.
Montesinos, E. 2003. Development, registration and commercialization of microbial pesticides for plant protection. Int Microbiol (2003) 6: 245–252 DOI 10.1007/s10123-003-0144-x.
Mutala’liah, Indarti S , Wibowo A. 2019. Short Communication: The prevalence and species of root-knot nematode which infect on potato seed in Central Java, Indonesia. Biodiversitas 20: 11-16.
Nasahi, C., Widiantini, F., Yulia, E., Meliansyah, R., Rasisetyo, P. 2016. Isolasi dan deteksi potensi aktinobacteria endofit dalam mengendalikan penyakit busuk pangkal batang pada tanaman kelapa sawit (Ganoderma boninense Pat.). Di Dalam: Joko T, editor. Prosiding Seminar Nasional Pengendalian Penyakit pada Tanaman Ramah Lingkungan II, 2016 Agustus 27. Yogyakarta (ID): Perhimpunan Fitopatologi Indonesia. hlm. 66–78.
Nasir, N., Pittaway, P.A., Pegg, K.G., et al. 1999. A pilot study investigating the complexity of Fusarium wilt of bananas in West Sumatra, Indonesia. Australian Journal of Agricultural Research 50: 1279–1283.
Nelson, G.A. 1982. Corynebacterium sepedonicum in potato: effect of inoculum concentration on ring rot symptoms and latent infection. Can J Plant Pathol 4:129–133.
Nion, Y.A., and Toyota, K. 2008. Suppression of bacterial wilt and fusarium wilt by Burkholderia nodosa strain isolated from Kalimantan soils, Indonesia. Microbes Environ. Vol. 23, No.2, 134-147.
Noble, R., E. Coventry. 2005: Suppression of soil-borne plant diseases with composts: a review. Biocontrol Sci. Technol. 15, 3-20.
Nugrahaeni, N. 2011. Pemuliaan kacang tanah untuk ketahanan terhadap layu bakteri Ralstonia di Indonesia. Bul. Palawija. No. 21:1–12.
Paterson, R.R.M. 2019. Ganoderma boninense Disease of Oil Palm to Significantly Reduce Production After 2050 in Sumatra if Projected Climate Change Occurs. Microorganisms, 7, 24; doi:10.3390/microorganisms7010024.
Pedai, T., Hadisutrisno, B., Priyatmojo, A. 2015. Utilization of Arbuscular Micorrhizal Fungi To Control Fusarium Wilt Of Tomatoes. Jurnal Perlindungan Tanaman Indonesia, Vol. 19, No. 2, 2015: 89–93.
Pimentel, D., Cooperstein, S., Randell, H., Filiberto, D., Sorrentino, S., Kaye, B., Nicklin, C., Yagi, J., Brian, J., & O’Hern, J., Habas, A., Weinstein, C. 2007. Ecology of Increasing Diseases: Population Growth and Environmental Degradation. Hum. Ecol. 35:653–668 DOI 10.1007/s10745-007-9128-3.
Postma, J., M. Montanari, M, Van Den Boogert, P.H.J.F. 2003: Microbial enrichment to enhance the disease suppressive activity of compost. Eur. J. Soil Biol. 39, 157-163.
Price, T.V. 2000. Plant parasitic nematodes. Prosiding pelatihan nematologi. Jakarta. 16-30 Juli 2000. Pusat Karantina Pertanian. Jakarta. Hal 27-34.
Prihatna, C., Barbetti, M.J., and Barker, S.J. 2018. A novel tomato Fusarium wilt tolerance gene. Front. Microbiol. 9: 1226. doi: 10.3389/fmicb.2018.01226.
Pugliese M, Benetti A, Gilardi G, Gullino ML, Garibaldi A. 2014. Control Of Soil-Borne Diseases by Different Composts In Potted Vegetable Crops. Commun Agric Appl Biol Sci. 2014;79(2): 37-40.
Rees, R.W., Flood, J., Hasan, Y., Wills, M.A., and Cooper, R.M. 2012. Ganoderma boninense basidiospores in oil palm plantations: evaluation of their possible role in stem rots of Elaeis guineensis. Plant Pathology 61, 567–578.
Rejeki Siti Ferniah, Rina Sri Kasiamdari, Achmadi Priyatmojo and Budi Setiadi Daryono. (2018). Resistance response of chilli (Capsicum annuum L.) F1 to Fusarium oxysporum involves expression of the CaChi2 gene. Tropical Life Sciences Research 29(2): 29–37. https://doi.org/10.21315/tlsr2018.29.2.3.
Riska, J., Hermanto, C. 2012. Hubungan antara tingkat konsentrasi inokulum Fusarium oxysporum f. sp. cubense VCG 01213/16 dengan 77 perkembangan penyakit layu pada kultivar pisang rentan. J Hort 22(2): 156-164.
Ryckeboer, J., Mergaert, J., Vaes, K., Klammer, S., De Clercq, D., Coosemans, J., Insam, H., Swings, J. 2003. A survey of bacteria and fungi occurring during composting and self-heating processes. Annals of Microbiology, 53 (4), 349-410.
Safitri, D., Soekarno, B.P.W., Achmad, Surono. 2012. Pemanfaatan bakteri endofit untuk meningkatkan ketahanan lada terhadap serangan Phytopthora capsici Leon penyebab penyakit busuk pangkal batang (Utilization of endophytic bacteria to increase pepper plant resistance to attack of Phytopthora capsici Leon causes stem rot disease). Proceeding of Seminar Nasional Mikologi, Universitas Jenderal Soedirman, Indonesia (National Seminar on Mycology, Jenderal Soedirman University, Indonesia), p. 489-499.
Sakpetch, P., H-Kittikun, A., Kuwahara, Y., Komeda, H., Asano, Y., 2018. Isolation of indigenous antagonistic microorganism to inhibit Rigidoporus microporus and other plant pathogens and analysis of the bioactive compounds. Biological Control, 124, 53–60.doi:10.1016/j.biocontrol.2018.01.007.
Schell MA. 2000. Control of virulence and pathogenicity genes of Ralstonia solanacearum by an elaborate sensory network. Annu Rev Phytopathol 38: 263-292.
Setyorini, D., R. Saraswati, dan E.K. Anwar. 2006. Kompos. P: 11 – 40. In R.D.M. Simanungkalit, D.A. Suriadikarta, R. Saraswati, D. Setyorini, dan W. Hartatik. Pupuk organic dan pupuk hayati. Balai Besar Litbang Sumberdaya Lahan Pertanian.
Simanungkalit, R.D.M., Ardi, D., Saraswati, R., Setyorini, D., Hartatik, W. 2006. Pupuk Organik dan Pupuk Hayati. Balai Besar Penelitian dan Pengembangan Sumberdaya Lahan Pertanian, Bogor. 14p.
Soekarno, B.P.W., Surono, Hendra. 2013. Optimalisasi peran kompos bioaktif dengan penambahan asam humat dan asam fulvat untuk meningkatkan ketahanan tanaman mentimun terhadap serangan Pythium sp. (Optimizing the role of bioactive compost with the addition of humic acid and fulvic acid to increase the cucumber plant resistance against Pythium sp.). Bionatura-Jurnal Ilmu-ilmu Hayati dan Fisik, Vol. 15, No. 1, March 2013: 35 – 43.
Soekarno, B.P.W., Surono, Rahmania, A. 2012. Keefektifan asam humat dan bakteri aktivator pada kompos untuk pengendalian rebah kecambah oleh Schlerotium rolfsii Sacc. pada Kacang Tanah (The effectiveness of humic acid and activator bacteria in the compost to control damping off disease caused by Schlerotium rolfsii Sacc. in peanut plant). Proceeding of Seminar Nasional Mikologi, Universitas Jenderal Soedirman, Indonesia (National Seminar on Mycology, Jenderal Soedirman University, Indonesia), p. 472-481.
Soekarno, B.P.W., Surono, Susanti. 2014. Formulasi Pelet Berbahan Aktif Trichoderma sp. untuk Pengendalian Penyakit Rebah Kecambah (Pythium sp.) pada Tanaman Mentimun (Pellet Formulation with Active Material of Trichoderma sp. for Controlling Damping Off Disease in Cucumber Plants). Jurnal Fitopatologi Indonesia (Indonesian Journal of Phytopathology) : Volume 10, Nomor 5, p 153–159, DOI: 10.14692/jfi.10.5.153.
Subowo, Purwani, J., and Rochayati, S. 2014. Biofertilizer Development Prospects and Challenges for Improved Soil Fertility. Jurnal Sumberdaya Lahan Vol 7 No. 1: 15-26.
Supyani and Widadi, S.. 2015. Hypovirulent Isolates of Fusarium Collected From Chili Crops in Boyolali Regency, Central Java, Indonesia. Agrivita Volume 37 No. 1: 67-74. http://dx.doi.org/10.17503/Agrivita-2015-37-1-p067-074.
Surono and Narisawa, K. (2017). The dark septate endophytic fungus Phialocephala fortinii is a potential decomposer of soil organic compounds and a promoter of Asparagus officinalis growth. Fungal Ecol. 28, 1–10. doi: 10.1016/j.funeco.2017.04.001.
Surono and Narisawa, K. (2018). The inhibitory role of dark septate endophytic fungus Phialocephala fortinii against Fusarium disease on the Asparagus officinalis growth in organic source conditions. Biological Control 121: 159-167. https://doi.org/10.1016/j.biocontrol.2018.02.017.
Surono, Soekarno, B.P.W., Alfian Muri L. 2012. Controlling Fusarium damping of on tomato using bioactive compound enriched by activator microbe and humic acid. As. J. Food Ag-Ind., 5(06), 476-484.
Surono. 2017. The role of dark septate endophytic fungus, Phialocephala fortinii, on promoting Asparagus officinalis growth under various stressed conditions. Doctoral Thesis, Tokyo University of Agriculture and Technology, Japan.
Surono. 2018. Isolation and selection of dark septate endophytic fungi (DSE) to promote rice growth in acidic and blast disease (Pyricularia oryzae) stress conditions. Final Report of SMARD Research Grant, the Indonesian Agency for Agricultural Research and Development (IAARD), the Ministry of Agriculture, INDONESIA, pp 71.
Syafi'i, D.D., Lisnawita, Hasanudin. 2018. Distribution of Potato Cyst Nematode in Wonosobo and Banjarnegara, Central Java. Volume 14, Nomor 4, Juli 2018 Halaman 111–119 DOI: 10.14692/jfi.14.4.111.
Tiquia, S.M., Michel, F.CJr. 2002. Bacterial Diversity in Livestock manure compost as characterized by terminal restriction fragment length polymorphisms (T-RFLP) of PCRamplified 16S rRNA gene sequences.
Widiantini, F., Nasahi, C., Yulia, E., Noviyawati, S. 2018. Potency of Endophytic Bacterial Secondary Metabolite to Inhibit Mycelium Growth of Ganoderma boninense. Volume 14, Nomor 3, Mei 2018 Halaman 104–109 DOI: 10.14692/jfi.14.3.104.
Yulianti, Nidar, T. 1999. Pertanian organik dan penyakit tanaman. Prosiding: Kongres Nasional XV dan Seminar Ilmiah Perhimpunan Fitopatologi Indonesia. Universitas Jenderal SUdirman. Purwokerto, 2000. Hal.: 592.
Yuliar, Nion, Y.A., and Toyota, K. 2015. Recent Trends in Control Methods for Bacterial Wilt Diseases Caused by Ralstonia solanacearum. Microbes Environ. Vol. 30, No. 1, 1-11.
Zaffan, Z.R., Soekarno, B.P.W., Munif, A., and Surono. 2018. Potential of Indonesia’s Indigenous Dark Septate Endophytic Fungi To Control Fusarium Wilt In Vitro. 3nd International Conference on Tropical Biology Ecological Restoration in Southeast Asia: Conservation, Enhancement and Sustainable Use of Indigenous Tropical Flora and Fauna. SEAMEO BIOTROP, Bogor-Indonesia, 20-21 September 2018.