ABSTRACT
Agricultural biotechnology has played a significant role in productivity improvement and has potentials in coping with rising food demand and more intense impacts from climate change. Several countries in Asia have embraced modern agricultural biotechnology such as genetic engineering, marker-assisted selection and emerging tools such as gene editing in their national policy. While Thailand recognized and responded to the potentials of agricultural biotechnology earlier than its neighbors, the position of Thailand in implementing biosafety legislation and adopting genetic engineered crops is far behind other countries in the region. Recently, Thailand’s policies on biotechnology are still uncertain although it is embedded as one of the tools in promoting the fourth industrial revolution or the so-called Thailand 4.0 policy. This paper provides current situation on trade, production, research and development, and economic issues related to Thailand’s agricultural biotechnology. It is suggested that Thailand may need to review its current global situation and market signal to modern biotechnology and respond more actively, not only because neighboring countries have already adopted the transgenic technology and could unavoidably crosses the border, but also because the emerging technology such as gene editing can be more acceptable in the global market.
Keywords: GMOs, genetically modified organisms, transgenic, gene editing, biosafety, GM food
INTRODUCTION
Biotechnology is considered to be one of the important modern technologies used in agricultural development. Modern biotechnology in agriculture, in particular, refers to the application of genetic engineering (World Health Organization, 2014) and development of transgenic plants and animals e.g. genetically modified (GM) maize, the use of marker-assisted selection (MAS) for plant breeding, etc. Recent modern biotechnology includes gene editing by applying Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) in editing plant DNA. Modern biotechnology allows more precise breeding for desired traits and shortens the time of research and development. MAS, for example, could create economic benefits from shortening the breeding cycle (Esparanza et al., 2009; Pandey and Rajatasereekul, 1999). Thailand is one of the first countries in Asia to recognize the potential benefits of agricultural biotechnology. Thailand’s National Center for Genetic Engineering and Biotechnology (BIOTEC) was established in 1983, and the first field trials of GM crop in Thailand was granted to Flavr Savr tomato in 1994 (Napasintuwong, 2010). In Southeast Asia, the Philippines was the first country to approve the commercialization of GM crops (maize) in 2002, followed by Myanmar (bt cotton) in 2006, and Vietnam (GM maize) in 2015. Nevertheless, at present, Thailand still has not approved any commercialization of GM crops for cultivation, and all open field trials of GM crops were banned after 2001.
Currently, Thailand’s biotechnology policy has broadened the importance of biotechnology to other sectors beyond food and agriculture including medicine and health, bioenergy, and bio-based industry according to Thailand’s National Biotechnology Policy Framework (2012-2021) (National Science Technology and Innovation Policy Office, 2011). The policy provides a framework aiming to stimulate R&D and its applications of biotechnology by promoting private sector’s investments and deepening community engagement in biotechnology, and to strengthen the country’s competitiveness and self sufficiency in the areas where Thailand either has strong potentials and/or pressing needs. This policy also aims at transforming Thailand into the center of biotechnology in Asia where Thailand currently chairs the ASEAN subcommittee on biotechnology and is a regional contributor to the industry (Royal Thai Embassy, Washington D.C., 2015).
Furthermore, as Thailand is in the stage of the fourth industrial revolution, the Thai government has adopted the economic growth model known as "Thailand 4.0” focusing on the concept of inclusive, productive and green growth to enhance the country’s competitiveness and economic development out of middle income trap. Under this model, Thailand is undergoing a reform of existing first five S-Curve industries while promoting the five new industries as growth engines. Of which, agriculture and biotechnology, food, biofuels and biochemicals are included (Office of the National Economic and Social Development Board, 2017). Following Thailand 4.0, Agriculture 4.0 is the government’s project which aims to increase productivity by reducing inefficiencies, water consumption, chemicals, and negative impacts on environment and society. This project emphasizes using agricultural technologies for more efficient farming. Under Agriculture 4.0, crop improvement using biotechnology i.e. marker assisted section, genetic engineering, germplasm collection, and gene editing is one of technological advancements that the Minister of Science and Technology of the junta government considered focused areas (Meesincee, 2018).
Studies on the ex-ante economic impacts of GM crop adoption have shown that GM crops can be beneficial for Thailand such as reducing use of chemicals, improved yield and lower prices in case of papaya, cotton, soybean and cassava (Napasintuwong and Traxler, 2009; Napasintuwong and Traxler, 2007; Thammasat University Economic Academic Service Center. 2014). Based on current biotechnology-related policies, this paper will focus on agricultural sector and provide history and current situations on technology development and trade of GM plants and GM food.
HISTORY OF AGRICULTURAL BIOTECHNOLOGY REGULATIONS IN THAILAND
Thailand was one of the first countries in the region to develop biotechnology industries. The establishment of the National Center for Genetic Engineering and Biotechnology (BIOTEC) under the Ministry of Science, Technology and Energy in 1983 marked a significant milestone for biotechnology development. Thailand was also the first country in the region to adopt national biosafety guidelines for both laboratory work and field testing and planned release in 1992. While BIOTEC served as the coordinating body and secretariat for biosafety regulation, in 1993, the National Biosafety Committee (NBC) was established, and later on, many Institutional Biosafety Committees (IBCs) were established by various research and academic institutes throughout Thailand (National Center for Genetic Engineering and Biotechnology, 2010). The NBC is no longer active and hence, the review of biosafety issues for GM plants and animals is currently being conducted by the Technical Biosafety Committee, an adhoc technical advisor of BIOTEC (Narkprasert and Thammakijjawat, 2018). Since then, there were several institutional reforms and restructuring and a series of policies and initiatives have been formulated and implemented by various government institutions.
The Department of Agriculture (DOA) with technical recommendations from NBC, granted a permission for the first field trial of GM crop to Flavr Savr tomato in 1994. Subsequently, field trials of GM crops were permitted for GM-cotton with toxin gene from Bacillus thuringenesis (Bt-cotton) in 1996, Bt-corn in 1997, and GM papaya in1997 (Napasintuwong, 2010; National Center for Genetic Engineering and Biotechnology, 2010). However, due to intensifying pressure from activists, on April 3, 2001 the government Cabinet decided to prohibit all open field trials of transgenic plants in Thailand until the National Biosafety Law is formulated and implemented. All field trials conducted for research purposes can only be implemented in confined areas. The ban on GM crop field trials was again revoked by the government Cabinet on 25 December 2007 under a case-by-case approval by the cabinet. Nevertheless, the requirements were considered restrictive e.g. it has to be in the fields owned by public institutes and the environmental and health impact assessment report has to be submitted for public hearing to local community, academia and NGOs; thus, no GM crops have been approved for field trials since 2003. Monsanto Thailand had planned to conduct a field trial for herbicide-resistant NK603 maize at Naresuan University, a public university, in 2013, but after the university changed its mind on hosting the project, Monsanto has not found other institute to host the this field trial (USDA Foreign Agricultural Service, 2018). Syngenta Thailand and Pioneer Thailand have reportedly discontinued their projects to conduct greenhouse trials of GM maize.
However, a series of biotechnology policies and initiatives have been continued to be generated and implemented. For example, the National Science, Technology and Innovation Policy Office (STI) and BIOTEC initiated the National Biotechnology Policy Frameworks for 2004-2009 and for 2012-2021 that aim to develop strategic planning, establish future R&D, and enhance the country’s ability to access new technologies and applications of biotechnology. Under National Biotechnology Policy Frameworks 2012-2021, agriculture and food sector is one of the four identified target industries. The goals were to advance market competiveness and strengthen agricultural sustainability and farmer livelihoods by increasing quality, productivity and innovation while reducing costs and responding to climate change (National Science Technology and Innovation Policy Office, 2011). Under the policy framework in regards to agriculture and food sector, the goals aim to:
- Improve crops cultivation and livestock by increasing productivity, resistance to pests and diseases, and meeting emerging industrial demands such as high-starch and fine pellet cassava or high-protein and antioxidant-rich rice, adapting to changing climatic conditions such as developing drought-resistant rubber;
- Improve agricultural inputs e.g. diversifying and increasing productivity of microorganism for soil nourishment through modifying organic fertilizers, pesticides and livestock food supplements to reduce antibiotics, and by developing easy-to-use vaccines and test kits for accurate disease diagnosis; and
- Increase agricultural and food production value by utilizing farm wastes as inputs for other industries such as sweeteners, bioenergy, biopolymers and other biochemical products as well as food supplements such as dissolvable fibers, low-calorie food, fat substitutes and biochemical substances.
These goals and associated strategies were perceived as not adequately addressing the needs of small-scale farmers. It was suggested that perhaps in the future it might be useful to review existing and potential roles of biotechnology in applications, for example, plant-breeding for drought, flood and salinity tolerance, and bio-fertilizers that are most needed by small-scale farmers and put measures in the implementation plan (United Nations Conference on Trade and Development, 2015).
The obstacle to the advancement in genetic engineering R&D in agriculture was due to the lack of national biosafety establishment. The national biosafety guidelines for laboratory testing, field testing and planned release of GMOs were created since 1992, and on February 8th 2006, Thailand became the 128th member country of the Cartagena Protocol on Biosafety (National Center for Genetic Engineering and Biotechnology, 2010). The draft national biosafety policy for Thailand was produced and submitted to the Parliament, but it has never been passed into law. According to the agreement by government Cabinet in 2007, the proposed Biosafety Act legislation will provide the legal framework regulating the use of agricultural biotechnology including research, field trials, and commercialization. Nevertheless, the support of Biosafety law is perceived by activists as the gateway to deregulate GM commercial production. As a result of several activists’ movements, after receiving approval from the Cabinet, the draft Biosafety Act was rejected by the Prime Minister in November 2015, stating that he did not see the legislation providing any benefit to Thailand (USDA Foreign Agricultural Service, 2018). On 1 November 2016, the Chairman of National Legislative Assembly’s (NLA)’s Science, Telecommunication, and Public Communication Committee created a new subcommittee to draft a new Biosafety Act. A revised draft was completed on 27 December 2016. The Ministry of Natural Resources and Environment (MONRE) has been nominated to combine the draft Biosafety Law with the draft Biodiversity Law. This draft legislation is still under review by MONRE and still has not been submitted to the Cabinet for approval (USDA Foreign Agricultural Service, 2018). As GM technology is a controversial issue, and public opinion is very divisive (Napasintuwong, 2010), Thailand has taken the application of genetic engineering technology in agriculture a cautious delay.
Moreover, as gene editing technology emerged, in particular CRISPR-Cas 9 which allows genetic information to be inserted, deleted, or altered at particular loci of the genome was considered most precise and efficient, the potential benefits, concerns over risks, and public acceptance towards gene editing might be different. CRISPR-Cas 9 is being applied to several areas of agricultural sector such as virus-resistant plant development, physical and chemical resistance crops, nutritional improvement, and manipulating production of bioactive compounds to mutate rice that increase in tillering, created flavored yeast to eliminate the use of hops in fermentation (Eş et al., 2019), and considered promising potentials in agricultural development. GM crops have often been considered the change in genetic in a way that does not occur naturally through mating and/or national recombination and can be a result of an introduction of foreign genes to crops while gene editing does not introduce foreign genes to the same species; thus, public opinion towards gene editing applied in agriculture and food industry tend to show more acceptance than GM technology (Shew et al., 2018). It might also be appropriate to review these issues and revise the draft biosafety policy before its resubmission to Parliament (United Nations Conference on Trade and Development, 2015).
AGRICULTURAL BIOTECHNOLOGY RESEARCH IN THAILAND
The availability of data on the application of biotechnology in agricultural research and development (R&D) are very limited especially after the delay of Biosafety legislation and prohibition of open field trials of GM crops. Nevertheless, the Gross Expenditure in Research and Development (GERD) in Thailand shows a continuously increasing trend in the past decade, particularly in the field of agricultural and natural sciences (Figure 1a and 1b). The private sector shows a significant increasing role in R&D in the past few years (Figure 1c and 1d). Starting in May 2019, the Thai government has restructured the research and funding agencies to combine higher education institutes, research institutes and Ministry of Science and Technology into Ministry of Higher Education, Science, Research and Innovation to empower the research capacity and human capacity development in the same direction as the national policy and strategic plan. With this new R&D infrastructure restructuring, the R&D performed by public institutes and higher education institutes might be reinforced.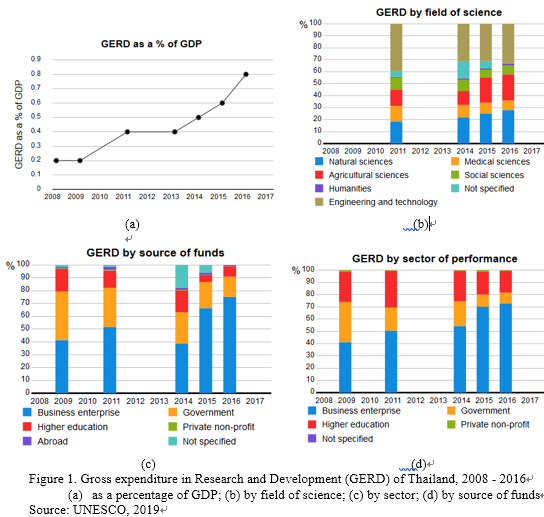
Because of stringent regulations of open field trials of GM crops even after the permission for field trials of GM crops that was reinstituted in 2007, R&D efforts for developing genetically modified crops have almost completely halted. The reported status of R&D of GM crops is summarized in Table 1.
Table 1. Status of research and development of GM crops in Thailand
|
Crop/Variety
|
Institute
|
Traits
|
Location
|
Year
|
|
Undergone Biosafety Test
|
|
Papaya/Khakdam and Khaknuan
|
DOA
|
Papaya Ringspot Virus (PRSV) resistant
|
Khon Kaen Horticultural Research Station
|
1997
|
|
Papaya/Khakdam and Khaknuan
|
BIOTEC
|
Papaya Ringspot Virus (PRSV) resistant
|
KU, KPS
|
1997
|
|
Papaya/Khakdam
|
BIOTEC
|
Delay ripening
|
KU, KPS
|
1999
|
|
Awaiting for Biosafety Test
|
|
Chili Pepper/Bang Chang
|
KU
|
Chilli vein-bandin gmottle virus(CVbMV) resistant
|
KU, KPS
|
1995-1998
|
|
Pineapple/Phuket
|
Rajamangala University of Technology Srivijaya, Nakhon Si Thammarat campus
|
Herbicide (bialaphos) tolerant
|
Rajamangala University of Technology Srivijaya, Nakhon Si Thammarat campus
|
2000
|
|
Tomato/VF134-1-2
|
BIOTEC
|
Tomato yellow leaf curl Thailand virus resistant and marker-free
|
KU, KPS
|
1999
|
|
Developing prototype crop
|
|
Dendrobium Orchid/Khao Sanan, Jaquelyn Thomas and Earsakul
|
KU
|
Color diversification
|
Faculty of Science, KU
|
1995
|
|
Oil palm
|
DOA
|
Perfect flower
|
Biotechnology Research and Development Office, DOA
|
n/a
|
|
Cotton
|
KU, KPS
|
Ballworm resistant
|
KU, KPS
|
1995-2001
|
|
Aquatic plants
|
KU, KPS
|
Fluorescent
|
KU, KPS
|
1996
|
|
Eucalyptus
|
KU, KPS
|
Reduced lignin
|
KU, KPS
|
1996
|
Source: Biosafety Clearing House of Thailand, 2009
Note: BIOTEC = National Center for Genetic Engineering and Biotechnology; DOA = Department of Agriculture; KU = Kasetsart University; KPS = Kampangsan campus
Presently the research on agricultural biotechnology is entirely done public institutes such as Biotechnology Research and Development Office under DOA, BIOTEC who hosts agricultural biotechnology research units i.e. Genome Research Unit, Animal Biotechnology Research Unit, and Food Biotechnology Research Unit (National Center for Genetic Engineering and Biotechnology, 2018), and other main public universities such as Rice Gene Discovery Laboratory, a collaboration between BIOTEC and Kasetsart University; Shrimp Molecular Biology and Biotechnology Laboratory, a multidisciplinary laboratory under Faculty of Science, Mahidol University and BIOTEC. The potential for molecular breeding research by public research institutes remains limited to the laboratory level. Nevertheless, biotechnology research especially molecular breeding at a laboratory scale of important crops has continued.
The Center for Agricultural Biotechnology at Kasetsart University, one of the founding institutes of Center of Excellence on Agricultural Biotechnology, a network of ten important higher education institutes in agriculture in Thailand, continues to receive a support from the Science and Technology Postgraduate Education and Research Development Office, Office of Higher Education. The center graduated 623 Masters and 177 Ph.D. in agricultural biotechnology to date (Center for Agricultural Biotechnology, 2010). Not only that it offers a higher education focusing on agricultural biotechnology, it provide services such as DNA sequencing and conduct research in the area of agricultural biotechnology such as MAS applied in plant breeding. The center uses a multidisciplinary approach using present state-of-the-art biology and biotechnology tools to address issues of efficiency improvement in the production of crops, animal and bio-energy. Some of research areas include using DNA, cellular tissue and biosystem and employing cumulative knowledge in biophysics, biochemistry and ecophysiology of agricultural science to identify the molecular markers for acquired traits for animal and crop improvement programs, developing procedures and test-kits for the identification of plant animal diseases and food contamination, and incorporating desirable genes in the production of vaccines and transgenic organisms.
Rice Science Center and Rice Gene Discovery Unit at Kasetsart University continuously conduct research using modern biotechnology. Key research areas include functional genomics, gene discovery and map-based cloning, molecular genetics, bioinformatics, and molecular breeding. This research unit has key success stories in using biotechnology as follows: (National Center for Genetic Engineering and Biotechnology, 2018).
- The research unit on behalf of The National Science and Technology Development Agency of Thailand received a US patent for ‘transgenic rice plants with reduced expression of Os2AP and elevated levels of 2-acetyl-1-pyroline which refers to the genes controlling the fragrance of Hom Mali or Jasmine Rice in June 2009 (Napasintuwong, 2011). This discovery of genes also obtained patents in the Australia, Japan, Vietnam, France and EU and patent pending in China, the Philippines and India.
- RiceGeneTresher: a web-based public resource for the rice genomic and bioinformatics software and platform for mining genes underlying quantitative trait locus (QTL) in the rice genome to deliver customized set of biological data on rice.
- Release improved rice varieties such as registered RD51 (flash flooding tolerance), Kaew Kaset (blast resistance and non-photoperiod sensitivity), Homcholasit (flash flooding tolerance and non-photoperiod sensitivity), Thanyasirin (blast resistant glutinous rice) and glutinous rice with blast and bacterial blight resistance.
Furthermore, the unit has implemented molecular rice breeding programs in the Mekong Region in 2004. A program aims to develop and enhance capacity building in MAS technology for rice breeders in Laos, Cambodia, Myanmar and Thailand. The Rice Science and Rice Gene Discovery Unit released Rice Berry, a purple rice that has good cooking quality and is rich in antioxidant. Rice Berry became very popular in domestic market among health-concerned consumers, and is currently being exported. The development of Rice Berry uses MAS from a cross of Hom Mali rice and black rice. Unlike previous popular rice varieties, Rice Berry is not developed by the Rice Department, a dominant public research institute in rice breeding. This shows that biotechnology is an advanced tool that could help addressing production problems and respond more promptly to changing market demand.
To encourage the R&D using biotechnology, the Thailand Board of Investment (BOI) provides incentives for the use of biotechnology by given eight year corporate income tax exception (no cap) and exemption of import duty on raw or essential materials and machinery used in manufacturing export products of following activities (Thailand Board of Investment, 2017):
- Research and development activity and/or manufacturing of seed industry, improvement of plants, animals, microorganisms using biotechnology, biopharmaceutical agents using biotechnology, diagnostic kits for health, agriculture, food and environment, biomolecules and bioactive substances using microorganisms, plant cells and animal cells.
- Manufacture of raw materials and/or essential materials for molecular biological research and development, experiments, testing or quality control services and/or production of biological substances.
- Biological substance analysis and/or synthesis services and/or quality control services and/or product validation services.
THAILAND’S REGULATIONS ON GM COMMODITY TRADE
Although Thailand does not allow commercial production of GM crops, it does allow the importation of GM food. Under the existing Plant Quarantine Act 1964, a list of 40 plant species known to have undergone genetic transformation in the world was added to prohibited list on 17 March 2000. On 14 October 2003, additional 49 transgenic plant varieties were added to prohibited list except for processed food (Napasintuwong, 2010). As the majority of soybeans, maize and cotton production by major exporters are transgenic, and Thailand is a trade deficit on these commodities; therefore, GM soybeans and GM maize for feed and industrial uses, and GM cotton lint are not prohibited items for imports. Thailand’s imports of soybeans and cotton from the U.S. totaled US$ 663 million in 2017, nearly all of which are GM products (USDA Foreign Agricultural Service, 2018). Furthermore, the regulation gives exemption to importation of processed food products containing GM materials.
Depending on trading partners, there are two main international trade frameworks in the context of GM products. The WTO framework is based upon a scientific evidence but not specific to biotechnology or GM products; whereas the Cartagena Protocol on Biosafety specifically targets at GM commodities on the socioeconomic and environmental considerations. Trade agreements related to GM products under WTO framework include Sanitary and Phytosanitary Measures (SPS), Technical Barriers to Trade (TBT) and the General Agreement on Tariffs and Trade (GATT), and Trade-Related Aspects of Intellectual Property Rights (TRIPS). In 2003, a joint program of WHO and the FAO--Codex Alimentarius Commission initiated food safety guidelines to provide international consistency in the assessment of GM products. The Codex principles do not have a compulsory effect on national legislations, but are referred to and often used as a reference in the case of trade disputes (World Health Organization, 2005). Codex assessment guidelines are based on scientific data such as chemicals, toxicological, and nutritional evaluations of the GM products and their conventional counterparts (Codex Alimentarius, 2009); thus, if GM food can be demonstrated as “substantially equivalent” to existing food or food counterpart, it can be regarded as being as safe. The Cartagena Protocol on Biosafety, on the other hand, uses the “precautionary approach” which institutes the right of a country to take into account socioeconomic considerations arising from the impact of GM products on the conservation and sustainable use of biodiversity, especially with regard to the value of biodiversity to indigenous and local communities, and the lack of scientific evidences shall not prevent a country from taking a decision, as appropriate, with regard to the import of GM products in order to avoid or minimize such potential adverse effects (Zarrilli, 2005).
WTO agreements commonly take Codex Alimentarius as a reference while the Cartagena Protocol put emphasis on local conservation and sustainable use of biodiversity, thus, countries standing on different ground of GM agreement may encounter a trade dispute on GM products. Major producers and traders of GM commodities such as Argentina, Australia, Canada, and the US do not ratify Cartagena Protocol while GM opposing countries such as the EU, Japan, South Korea, the UK and most developing countries do (Convention on Biological Diversity, 2018). Before Thailand became a member of Cartagena Protocol on Biosafety in 2006, in 2000 there was a GM trade dispute for import restriction of canned tuna fish in soybean oil from Thailand to Egypt filed for WTO consultation. More recently, according to the European Union Rapid Alert System for Food and Feed (RASFF), more than 40 shipments of papayas originating from Thailand were detected positive for GM contamination and rejected from 2013-2017 (USDA Foreign Agricultural Service, 2018). Since 2014, the DOA regulated that all exports of fresh or dried papaya or food products containing papaya to the EU and Japan are subject to GM detection testing prior to shipping. Furthermore, in 2016, formal criteria must be met for exporters of Thai fresh papaya in order to export to the EU, Switzerland, Norway, Iceland, China, and Japan (USDA Foreign Agricultural Service, 2018).
GM FOOD LABELING
GM food labeling regulations varied significantly across countries (Gruere and Rao, 2007). Codex Alimentarius Commission considers that different countries may make their own decisions on whether or not to label GM foods, and emphasizes that labelling arrangements should be in conformity with the provisions promulgated by the Codex to avoid potential trade issues (Centre for Food Safety, 2019). Policies on GM food labelling vary significantly in different countries. While some countries such as Australia, China, the E.U., New Zealand, Japan, South Korea, Switzerland, Taiwan and Thailand adopted mandatory labeling of GM food, GM food labeling in others countries such as Canada, Hong Kong and the U.S. is voluntary. The voluntary labeling approach generally requires that only GM food that is significantly different from its conventional counterpart needs to be labelled. The mandatory labeling approach can be either “pan-labeling” or “labeling for designated products only”. The “pan-labeling” requires that any food products, which contain GM materials exceeding a threshold level or are significantly different as a result of genetic modification, must be labelled. The “labelling for designated products only” requires that only the designated GM products need to be labelled (Centre for Food Safety, 2019). Furthermore, the threshold of contamination, whether it is enforced on process or product, the product coverage and exemption also varied across countries.
In December 2018, the U.S., one of the major producers of GM crops, announced the first regulation for GM food labeling which will be in effect on 1 January 2022. Under National Bioengineered Food Disclosure Standard, certain products made from the 13 GM crops and foods on the USDA’s list including papaya and pineapple, however, do not require labeling. Refined foods derived from GM crops that do not contain detectable modified genetic material are not required for GM labeling (USDA Agricultural Marketing Service, 2018).
Because producers and sellers of GM foods have more information about sources of product materials more than consumers, the market of GM foods is asymmetric information without mandatory labeling. In Thailand, the Ministry of Public Health’s Notification on GM Food Labeling 2002 adopted mandatory labeling for designated products only. It requires that 22 food items containing ingredients derived from GM soybean (and its products) and maize (and its products) more than 5% of the total weight in the top three components by weight and containing more than 5% of GM components of each ingredient are subject to mandatory GM food labeling (Ministry of Public Health, 2002). The list of 22 products subject to mandatory positive labeling includes 1) soybeans, 2) cooked soybeans, 3) roasted soybeans, 4) bottled or canned soybeans or soybeans contained in retort pouch, 5) natto, 6) miso, 7) tofu or tofu fried in oil, 8) frozen tofu, 9) soybean gluten from tofu or its products, 9) soybean milk, 10) soybean flour, 11) food containing product(s) from 1 to 10 as the main ingredient, 12) food containing soybean protein as main ingredient, 13) food containing green soybean as main ingredient, 14) food containing soybean sprout as main ingredient, 15) corn, 16) popcorn, 17) frozen or chilled corn, 18) bottled or canned corn or corn contained in heat-treated pouch, 19) corn flour or cornstarch, 20) snack foods deriving from corn as main ingredient, 21) food containing product(s) from 15 to 20 as the main ingredient, and 22) food containing corn grits as main ingredient.
Positive GM labeling enforces that food containing only one main ingredient should include a statement of “genetically modified” in conjunction with, or in close proximity to, the name of the food such as “genetically modified corn” or “tofu produced from genetically modified soybean”. For multi-ingredient products, labels should include a statement of “genetically modified” in conjunction with, or in close proximity to, or under the names of top three main ingredients of the food product such as “genetically modified corn starch”. Unlike voluntary negative labeling that provides information about the absence of GM ingredients, the use of “GMO-free”, “non-GM”, “does not contain GM”, or other statements alike is prohibited in Thailand (Figure 2). The regulation, however, is not applied to small venders who produce and directly sell to consumers. Although Thailand does not allow negative labeling of GM food products, some products in the market were found to be labelled GM negatively (Figure 3). Krualee and Napasintuwong (2012) found that negative labeling may be more appropriate than positive labeling for designated products only for Thailand when the majority of consumers are averse to GM food and willing to pay less for GM contaminated products or if they consider negative health impacts a serious problem. The advantage of positive labeling for designated food products allows consumers to have information whether the designated food items contain GM materials and hence make informed choices, but it imposes significant costs of segregation, testing, certification and labeling to the food industry that may either be absorbed by the producers or passed on to the consumers Krualee and Napasintuwong (2012). Thus, should be weighed against the benefits to consumers.
Thai Food and Drug Administration is currently considering adopting a mandatory food safety dossier submission for food ingredients derived from GM crops. If this regulation is adopted, a positive list of approved GM events will be created and would be allowed to be used as food ingredients. The final rule is projected to be approved in late 2019 (USDA Foreign Agricultural Service, 2018).


CONCLUSION
As modern biotechnology has shown positive impacts in several countries, the adoption of GM crops has increased after its first commercialization in 1996. Countries in Southeast Asia including Myanmar, Philippines, and Vietnam have legalized GM maize production. Although Thailand started the field trials of GM crops as early as in the 1900s, but due to the lack of Biosafety legislation and a precautionary delay, presently there is no open field trials nor commercial production of GM crops. Undecided policies and regulations have prohibited the progress of the recognized potential benefits from transgenic technology even though the use of modern biotechnology such as Marker Assisted Selection and genomic and bioinformatics and others are being supported by the government. While restrictions on production and imports of GM crops continue, Thailand does allow importation of GM soybeans, GM maize, GM cotton lints, and GM processed food. At the same time, Vietnam and Myanmar have approved GM crop cultivation. As in the case of GM papaya contamination in papaya products from Thailand, it is likely that GM seeds will cross the border from neighboring countries and create difficulties to regulate unauthorized GM crops in the future unless Biosafety legislation is enacted. This paper has provided situations on R&D, regulations and status on GM crop and food products in Thailand. It is suggested that Thailand will have to consider changes for appropriate policies in response to changes in global biotechnology advancement.
REFERENCES
Biosafety Clearing House of Thailand. 2009. Status of research and development of genetically modified plants. Office of Natural Resources and Environmental Policy and Planning. ( http://bch-thai.onep.go.th/status_research_develop_plant.html; Accessed 20 May 2019)
Centre for Food Safety. 2019. International development in labelling of GM foods
(https://www.cfs.gov.hk/english/programme/programme_gmf/programme_gmf_gi_... Accessed 20 May 2019)
Convention on Biological Diversity. 2018. Parties to the Cartagena Protocol and its supplementary protocol on liability and redress. (https://bch.cbd.int/protocol/parties/; Accessed 20 May 2019)
Center for Agricultural Biotechnology. 2010. About the center.
(http://www.cab.ku.ac.th/CAB-2010-04/aboutme.php; Accessed 20 May 2019)
Codex Alimentarius Commission. 2009. Food derived from modern biotechnology. Second edition.
(http://www.fao.org/3/a1554e/a1554e00.htm; Accessed 20 May 2019)
Esparanza, V.L., Alpuerto, B., Norton, G.W., Alwang, J., and Ismail, M. 2009. Economic impact analysis of marker-assisted breeding for tolerance to salinity and phosphorous deficiency in rice. Review of Agricultural Economics 31(4): 779–92.
Eşa, I., Gavahianb, M., Marti-Quijalc, F.J., Lorenzod, J.M., Khaneghahe, A.M., Tsatsanisf, C., Kampranisg, S.C., and Barbac, F.J. 2019. Biotechnology Advances 37(3): 410-421.
Gruere, G. P., and Rao, S. R. 2007. A review of international labeling policies of genetically modified food to evaluate India’s proposed rule. AgBioForum 10(1): 51-64.
Krualee, S., and Napasintuwong, O. 2012. Consumers’ willingness to pay for non-GM food labeling in Thailand. International Food Research Journal 19(4): 1375-1382.
Meesincee, S. (2018). Adaptation of agriculture and food industry to Thailand 4.0. Dinner Talk organized by the Agricultural Economics Society of Thailand under Royal Patronage. 27 September 2018. Centara Grand at Central Ladprao, Bangkok.
Ministry of Public Health. 2002. Notification on labeling regulation of transgenic food or food derived from genetic engineering technique (No. 251) B.E. 2545 (2002). (in Thai)
Napasintuwong, O. (2011). The rice of rice biotechnology. Farm Policy Journal 8(1): 55-65.
Napasintuwong, O. (2010). The role of agricultural biotechnology policies in Thailand’s economy. Asian Biotechnology and Development Review 12(1): 1-19.
Napasintuwong, O., and Traxler, G. J. 2009. Ex-ante impact assessment of GM-papaya adoption in Thailand. AgBioForum 12(2): 209-217.
Napasintuwong, O. and Traxler, G.J. 2007. Ex-ante economic evaluation of transgenic plants adoption in Thailand. Kasetsart University Journal of Economics 14(2): 86-102. (in Thai).
Narkprasert, D., and Thammakijjawat, P. 2018. Country status report—Thailand. Regional Expert Consultation on Agricultural Biotechnology – Scoping Partnerships to Improve Livelihoods of
Farmers in Asia and the Pacific. Strategic Papers and Country Status Reports. Bangkok, Thailand; 29-31 May 2018.
National Center for Genetic Engineering and Biotechnology (BIOTEC). 2018. Research Unit.
(http://www.biotec.or.th/en/index.php/research/research-units; Accessed 20 May 2019)
National Center for Genetic Engineering and Biotechnology (BIOTEC). 2010. White paper: Updated Status and Perspective of Thailand on Research and Development of Modern Biotechnology and Biosafety Regulation. 3rd Edition. Technical Biosafety Committee, Thailand.
National Science Technology and Innovation Policy Office (STI). 2011. Thailand’s National Biotechnology Policy Framework (2012-2021). Ministry of Science and Technology, Thailand
Office of the National Economic and Social Development Board (NESDB). 2017. The 12th National Economic and Social Development Plan, 2017–2021. (http://www.nesdb.go.th; Accessed 20 May 2019) (in Thai)
Pandey, S., and Rajatasereekul, S. 1999. Economics of plant breeding: the value of shorter breeding cycles for rice in Northeast Thailand, Field Crops Research 64(1–2): 187–97.
Royal Thai Embassy, Washington D.C. (2015) Biotechnology. Industry in Thailand
(https://thaiembdc.org/thai-industries/; Accessed 20 May 2019)
Shew, A. M., Nalley, L. L., Snell, H. A., Nayga, R. M., Jr., and Dixon, B. L. 2018. CRISPR versus GMOs: Public acceptance and valuation. Global Food Security 19: 71-80.
Thammasat University Economic Academic Service Center. 2014. Study on the economic and social impacts of genetically modified crops in Thailand. Report submitted to National Science Technology and Innovation Policy Office. (in Thai)
Thailand Board of Investment. 2017. Thailand’s Bioeconomy Industry.
(https://www.boi.go.th/index.php?page=brochure; Accessed 20 May 2019)
UNESCO. 2019. Data for the sustainable development goals. Thailand—Science, technology and innovation indicators. Expenditure on R&D
(http://uis.unesco.org/en/country/th?theme=science-technology-and-innovat... Accessed 20 May 2019)
United Nations Conference on Trade and Development (UNCTD). 2015. Science, technology and innovation policy review – Thailand. United Nations, New York and Geneva.
USDA Agricultural Marketing Service. 2018. Bioengineered food disclosure and labeling.
(https://www.ams.usda.gov/rules-regulations/be; Accessed 20 May 2019)
USDA Foreign Agricultural Service. 2018. Thailand Agricultural Biotechnology Annual 2018. Global Agricultural Information Network (GAIN) Report Number: TH8166. 20 December 2018. (https://www.fas.usda.gov/data/thailand-agricultural-biotechnology-annual-1; Accessed 20 May 2019)
World Health Organization. 2014. Frequently asked questions on genetically modified foods.
(https://www.who.int/foodsafety/areas_work/food-technology/faq-geneticall... Accessed 20 May 2019)
World Trade Organization. 2005. Modern food biotechnology, human health and development: An evidence-based study. (https://apps.who.int/iris/handle/10665/43195; Accessed 20 May 2019)
Zarrilli, S. 2005. International trade in GMOs and GM products: National and multinational legal frameworks. United Nations Conference on Trade and Development. Policy Issues in International Trade and Commodities, Study Series No. 29.
|
Date submitted: May. 30, 2019
Reviewed, edited and uploaded: June. 13, 2019
|


Current Status of Agricultural Biotechnology in Thailand
ABSTRACT
Agricultural biotechnology has played a significant role in productivity improvement and has potentials in coping with rising food demand and more intense impacts from climate change. Several countries in Asia have embraced modern agricultural biotechnology such as genetic engineering, marker-assisted selection and emerging tools such as gene editing in their national policy. While Thailand recognized and responded to the potentials of agricultural biotechnology earlier than its neighbors, the position of Thailand in implementing biosafety legislation and adopting genetic engineered crops is far behind other countries in the region. Recently, Thailand’s policies on biotechnology are still uncertain although it is embedded as one of the tools in promoting the fourth industrial revolution or the so-called Thailand 4.0 policy. This paper provides current situation on trade, production, research and development, and economic issues related to Thailand’s agricultural biotechnology. It is suggested that Thailand may need to review its current global situation and market signal to modern biotechnology and respond more actively, not only because neighboring countries have already adopted the transgenic technology and could unavoidably crosses the border, but also because the emerging technology such as gene editing can be more acceptable in the global market.
Keywords: GMOs, genetically modified organisms, transgenic, gene editing, biosafety, GM food
INTRODUCTION
Biotechnology is considered to be one of the important modern technologies used in agricultural development. Modern biotechnology in agriculture, in particular, refers to the application of genetic engineering (World Health Organization, 2014) and development of transgenic plants and animals e.g. genetically modified (GM) maize, the use of marker-assisted selection (MAS) for plant breeding, etc. Recent modern biotechnology includes gene editing by applying Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) in editing plant DNA. Modern biotechnology allows more precise breeding for desired traits and shortens the time of research and development. MAS, for example, could create economic benefits from shortening the breeding cycle (Esparanza et al., 2009; Pandey and Rajatasereekul, 1999). Thailand is one of the first countries in Asia to recognize the potential benefits of agricultural biotechnology. Thailand’s National Center for Genetic Engineering and Biotechnology (BIOTEC) was established in 1983, and the first field trials of GM crop in Thailand was granted to Flavr Savr tomato in 1994 (Napasintuwong, 2010). In Southeast Asia, the Philippines was the first country to approve the commercialization of GM crops (maize) in 2002, followed by Myanmar (bt cotton) in 2006, and Vietnam (GM maize) in 2015. Nevertheless, at present, Thailand still has not approved any commercialization of GM crops for cultivation, and all open field trials of GM crops were banned after 2001.
Currently, Thailand’s biotechnology policy has broadened the importance of biotechnology to other sectors beyond food and agriculture including medicine and health, bioenergy, and bio-based industry according to Thailand’s National Biotechnology Policy Framework (2012-2021) (National Science Technology and Innovation Policy Office, 2011). The policy provides a framework aiming to stimulate R&D and its applications of biotechnology by promoting private sector’s investments and deepening community engagement in biotechnology, and to strengthen the country’s competitiveness and self sufficiency in the areas where Thailand either has strong potentials and/or pressing needs. This policy also aims at transforming Thailand into the center of biotechnology in Asia where Thailand currently chairs the ASEAN subcommittee on biotechnology and is a regional contributor to the industry (Royal Thai Embassy, Washington D.C., 2015).
Furthermore, as Thailand is in the stage of the fourth industrial revolution, the Thai government has adopted the economic growth model known as "Thailand 4.0” focusing on the concept of inclusive, productive and green growth to enhance the country’s competitiveness and economic development out of middle income trap. Under this model, Thailand is undergoing a reform of existing first five S-Curve industries while promoting the five new industries as growth engines. Of which, agriculture and biotechnology, food, biofuels and biochemicals are included (Office of the National Economic and Social Development Board, 2017). Following Thailand 4.0, Agriculture 4.0 is the government’s project which aims to increase productivity by reducing inefficiencies, water consumption, chemicals, and negative impacts on environment and society. This project emphasizes using agricultural technologies for more efficient farming. Under Agriculture 4.0, crop improvement using biotechnology i.e. marker assisted section, genetic engineering, germplasm collection, and gene editing is one of technological advancements that the Minister of Science and Technology of the junta government considered focused areas (Meesincee, 2018).
Studies on the ex-ante economic impacts of GM crop adoption have shown that GM crops can be beneficial for Thailand such as reducing use of chemicals, improved yield and lower prices in case of papaya, cotton, soybean and cassava (Napasintuwong and Traxler, 2009; Napasintuwong and Traxler, 2007; Thammasat University Economic Academic Service Center. 2014). Based on current biotechnology-related policies, this paper will focus on agricultural sector and provide history and current situations on technology development and trade of GM plants and GM food.
HISTORY OF AGRICULTURAL BIOTECHNOLOGY REGULATIONS IN THAILAND
Thailand was one of the first countries in the region to develop biotechnology industries. The establishment of the National Center for Genetic Engineering and Biotechnology (BIOTEC) under the Ministry of Science, Technology and Energy in 1983 marked a significant milestone for biotechnology development. Thailand was also the first country in the region to adopt national biosafety guidelines for both laboratory work and field testing and planned release in 1992. While BIOTEC served as the coordinating body and secretariat for biosafety regulation, in 1993, the National Biosafety Committee (NBC) was established, and later on, many Institutional Biosafety Committees (IBCs) were established by various research and academic institutes throughout Thailand (National Center for Genetic Engineering and Biotechnology, 2010). The NBC is no longer active and hence, the review of biosafety issues for GM plants and animals is currently being conducted by the Technical Biosafety Committee, an adhoc technical advisor of BIOTEC (Narkprasert and Thammakijjawat, 2018). Since then, there were several institutional reforms and restructuring and a series of policies and initiatives have been formulated and implemented by various government institutions.
The Department of Agriculture (DOA) with technical recommendations from NBC, granted a permission for the first field trial of GM crop to Flavr Savr tomato in 1994. Subsequently, field trials of GM crops were permitted for GM-cotton with toxin gene from Bacillus thuringenesis (Bt-cotton) in 1996, Bt-corn in 1997, and GM papaya in1997 (Napasintuwong, 2010; National Center for Genetic Engineering and Biotechnology, 2010). However, due to intensifying pressure from activists, on April 3, 2001 the government Cabinet decided to prohibit all open field trials of transgenic plants in Thailand until the National Biosafety Law is formulated and implemented. All field trials conducted for research purposes can only be implemented in confined areas. The ban on GM crop field trials was again revoked by the government Cabinet on 25 December 2007 under a case-by-case approval by the cabinet. Nevertheless, the requirements were considered restrictive e.g. it has to be in the fields owned by public institutes and the environmental and health impact assessment report has to be submitted for public hearing to local community, academia and NGOs; thus, no GM crops have been approved for field trials since 2003. Monsanto Thailand had planned to conduct a field trial for herbicide-resistant NK603 maize at Naresuan University, a public university, in 2013, but after the university changed its mind on hosting the project, Monsanto has not found other institute to host the this field trial (USDA Foreign Agricultural Service, 2018). Syngenta Thailand and Pioneer Thailand have reportedly discontinued their projects to conduct greenhouse trials of GM maize.
However, a series of biotechnology policies and initiatives have been continued to be generated and implemented. For example, the National Science, Technology and Innovation Policy Office (STI) and BIOTEC initiated the National Biotechnology Policy Frameworks for 2004-2009 and for 2012-2021 that aim to develop strategic planning, establish future R&D, and enhance the country’s ability to access new technologies and applications of biotechnology. Under National Biotechnology Policy Frameworks 2012-2021, agriculture and food sector is one of the four identified target industries. The goals were to advance market competiveness and strengthen agricultural sustainability and farmer livelihoods by increasing quality, productivity and innovation while reducing costs and responding to climate change (National Science Technology and Innovation Policy Office, 2011). Under the policy framework in regards to agriculture and food sector, the goals aim to:
These goals and associated strategies were perceived as not adequately addressing the needs of small-scale farmers. It was suggested that perhaps in the future it might be useful to review existing and potential roles of biotechnology in applications, for example, plant-breeding for drought, flood and salinity tolerance, and bio-fertilizers that are most needed by small-scale farmers and put measures in the implementation plan (United Nations Conference on Trade and Development, 2015).
The obstacle to the advancement in genetic engineering R&D in agriculture was due to the lack of national biosafety establishment. The national biosafety guidelines for laboratory testing, field testing and planned release of GMOs were created since 1992, and on February 8th 2006, Thailand became the 128th member country of the Cartagena Protocol on Biosafety (National Center for Genetic Engineering and Biotechnology, 2010). The draft national biosafety policy for Thailand was produced and submitted to the Parliament, but it has never been passed into law. According to the agreement by government Cabinet in 2007, the proposed Biosafety Act legislation will provide the legal framework regulating the use of agricultural biotechnology including research, field trials, and commercialization. Nevertheless, the support of Biosafety law is perceived by activists as the gateway to deregulate GM commercial production. As a result of several activists’ movements, after receiving approval from the Cabinet, the draft Biosafety Act was rejected by the Prime Minister in November 2015, stating that he did not see the legislation providing any benefit to Thailand (USDA Foreign Agricultural Service, 2018). On 1 November 2016, the Chairman of National Legislative Assembly’s (NLA)’s Science, Telecommunication, and Public Communication Committee created a new subcommittee to draft a new Biosafety Act. A revised draft was completed on 27 December 2016. The Ministry of Natural Resources and Environment (MONRE) has been nominated to combine the draft Biosafety Law with the draft Biodiversity Law. This draft legislation is still under review by MONRE and still has not been submitted to the Cabinet for approval (USDA Foreign Agricultural Service, 2018). As GM technology is a controversial issue, and public opinion is very divisive (Napasintuwong, 2010), Thailand has taken the application of genetic engineering technology in agriculture a cautious delay.
Moreover, as gene editing technology emerged, in particular CRISPR-Cas 9 which allows genetic information to be inserted, deleted, or altered at particular loci of the genome was considered most precise and efficient, the potential benefits, concerns over risks, and public acceptance towards gene editing might be different. CRISPR-Cas 9 is being applied to several areas of agricultural sector such as virus-resistant plant development, physical and chemical resistance crops, nutritional improvement, and manipulating production of bioactive compounds to mutate rice that increase in tillering, created flavored yeast to eliminate the use of hops in fermentation (Eş et al., 2019), and considered promising potentials in agricultural development. GM crops have often been considered the change in genetic in a way that does not occur naturally through mating and/or national recombination and can be a result of an introduction of foreign genes to crops while gene editing does not introduce foreign genes to the same species; thus, public opinion towards gene editing applied in agriculture and food industry tend to show more acceptance than GM technology (Shew et al., 2018). It might also be appropriate to review these issues and revise the draft biosafety policy before its resubmission to Parliament (United Nations Conference on Trade and Development, 2015).
AGRICULTURAL BIOTECHNOLOGY RESEARCH IN THAILAND
The availability of data on the application of biotechnology in agricultural research and development (R&D) are very limited especially after the delay of Biosafety legislation and prohibition of open field trials of GM crops. Nevertheless, the Gross Expenditure in Research and Development (GERD) in Thailand shows a continuously increasing trend in the past decade, particularly in the field of agricultural and natural sciences (Figure 1a and 1b). The private sector shows a significant increasing role in R&D in the past few years (Figure 1c and 1d). Starting in May 2019, the Thai government has restructured the research and funding agencies to combine higher education institutes, research institutes and Ministry of Science and Technology into Ministry of Higher Education, Science, Research and Innovation to empower the research capacity and human capacity development in the same direction as the national policy and strategic plan. With this new R&D infrastructure restructuring, the R&D performed by public institutes and higher education institutes might be reinforced.
Because of stringent regulations of open field trials of GM crops even after the permission for field trials of GM crops that was reinstituted in 2007, R&D efforts for developing genetically modified crops have almost completely halted. The reported status of R&D of GM crops is summarized in Table 1.
Table 1. Status of research and development of GM crops in Thailand
Crop/Variety
Institute
Traits
Location
Year
Undergone Biosafety Test
Papaya/Khakdam and Khaknuan
DOA
Papaya Ringspot Virus (PRSV) resistant
Khon Kaen Horticultural Research Station
1997
Papaya/Khakdam and Khaknuan
BIOTEC
Papaya Ringspot Virus (PRSV) resistant
KU, KPS
1997
Papaya/Khakdam
BIOTEC
Delay ripening
KU, KPS
1999
Awaiting for Biosafety Test
Chili Pepper/Bang Chang
KU
Chilli vein-bandin gmottle virus(CVbMV) resistant
KU, KPS
1995-1998
Pineapple/Phuket
Rajamangala University of Technology Srivijaya, Nakhon Si Thammarat campus
Herbicide (bialaphos) tolerant
Rajamangala University of Technology Srivijaya, Nakhon Si Thammarat campus
2000
Tomato/VF134-1-2
BIOTEC
Tomato yellow leaf curl Thailand virus resistant and marker-free
KU, KPS
1999
Developing prototype crop
Dendrobium Orchid/Khao Sanan, Jaquelyn Thomas and Earsakul
KU
Color diversification
Faculty of Science, KU
1995
Oil palm
DOA
Perfect flower
Biotechnology Research and Development Office, DOA
n/a
Cotton
KU, KPS
Ballworm resistant
KU, KPS
1995-2001
Aquatic plants
KU, KPS
Fluorescent
KU, KPS
1996
Eucalyptus
KU, KPS
Reduced lignin
KU, KPS
1996
Source: Biosafety Clearing House of Thailand, 2009
Note: BIOTEC = National Center for Genetic Engineering and Biotechnology; DOA = Department of Agriculture; KU = Kasetsart University; KPS = Kampangsan campus
Presently the research on agricultural biotechnology is entirely done public institutes such as Biotechnology Research and Development Office under DOA, BIOTEC who hosts agricultural biotechnology research units i.e. Genome Research Unit, Animal Biotechnology Research Unit, and Food Biotechnology Research Unit (National Center for Genetic Engineering and Biotechnology, 2018), and other main public universities such as Rice Gene Discovery Laboratory, a collaboration between BIOTEC and Kasetsart University; Shrimp Molecular Biology and Biotechnology Laboratory, a multidisciplinary laboratory under Faculty of Science, Mahidol University and BIOTEC. The potential for molecular breeding research by public research institutes remains limited to the laboratory level. Nevertheless, biotechnology research especially molecular breeding at a laboratory scale of important crops has continued.
The Center for Agricultural Biotechnology at Kasetsart University, one of the founding institutes of Center of Excellence on Agricultural Biotechnology, a network of ten important higher education institutes in agriculture in Thailand, continues to receive a support from the Science and Technology Postgraduate Education and Research Development Office, Office of Higher Education. The center graduated 623 Masters and 177 Ph.D. in agricultural biotechnology to date (Center for Agricultural Biotechnology, 2010). Not only that it offers a higher education focusing on agricultural biotechnology, it provide services such as DNA sequencing and conduct research in the area of agricultural biotechnology such as MAS applied in plant breeding. The center uses a multidisciplinary approach using present state-of-the-art biology and biotechnology tools to address issues of efficiency improvement in the production of crops, animal and bio-energy. Some of research areas include using DNA, cellular tissue and biosystem and employing cumulative knowledge in biophysics, biochemistry and ecophysiology of agricultural science to identify the molecular markers for acquired traits for animal and crop improvement programs, developing procedures and test-kits for the identification of plant animal diseases and food contamination, and incorporating desirable genes in the production of vaccines and transgenic organisms.
Rice Science Center and Rice Gene Discovery Unit at Kasetsart University continuously conduct research using modern biotechnology. Key research areas include functional genomics, gene discovery and map-based cloning, molecular genetics, bioinformatics, and molecular breeding. This research unit has key success stories in using biotechnology as follows: (National Center for Genetic Engineering and Biotechnology, 2018).
Furthermore, the unit has implemented molecular rice breeding programs in the Mekong Region in 2004. A program aims to develop and enhance capacity building in MAS technology for rice breeders in Laos, Cambodia, Myanmar and Thailand. The Rice Science and Rice Gene Discovery Unit released Rice Berry, a purple rice that has good cooking quality and is rich in antioxidant. Rice Berry became very popular in domestic market among health-concerned consumers, and is currently being exported. The development of Rice Berry uses MAS from a cross of Hom Mali rice and black rice. Unlike previous popular rice varieties, Rice Berry is not developed by the Rice Department, a dominant public research institute in rice breeding. This shows that biotechnology is an advanced tool that could help addressing production problems and respond more promptly to changing market demand.
To encourage the R&D using biotechnology, the Thailand Board of Investment (BOI) provides incentives for the use of biotechnology by given eight year corporate income tax exception (no cap) and exemption of import duty on raw or essential materials and machinery used in manufacturing export products of following activities (Thailand Board of Investment, 2017):
THAILAND’S REGULATIONS ON GM COMMODITY TRADE
Although Thailand does not allow commercial production of GM crops, it does allow the importation of GM food. Under the existing Plant Quarantine Act 1964, a list of 40 plant species known to have undergone genetic transformation in the world was added to prohibited list on 17 March 2000. On 14 October 2003, additional 49 transgenic plant varieties were added to prohibited list except for processed food (Napasintuwong, 2010). As the majority of soybeans, maize and cotton production by major exporters are transgenic, and Thailand is a trade deficit on these commodities; therefore, GM soybeans and GM maize for feed and industrial uses, and GM cotton lint are not prohibited items for imports. Thailand’s imports of soybeans and cotton from the U.S. totaled US$ 663 million in 2017, nearly all of which are GM products (USDA Foreign Agricultural Service, 2018). Furthermore, the regulation gives exemption to importation of processed food products containing GM materials.
Depending on trading partners, there are two main international trade frameworks in the context of GM products. The WTO framework is based upon a scientific evidence but not specific to biotechnology or GM products; whereas the Cartagena Protocol on Biosafety specifically targets at GM commodities on the socioeconomic and environmental considerations. Trade agreements related to GM products under WTO framework include Sanitary and Phytosanitary Measures (SPS), Technical Barriers to Trade (TBT) and the General Agreement on Tariffs and Trade (GATT), and Trade-Related Aspects of Intellectual Property Rights (TRIPS). In 2003, a joint program of WHO and the FAO--Codex Alimentarius Commission initiated food safety guidelines to provide international consistency in the assessment of GM products. The Codex principles do not have a compulsory effect on national legislations, but are referred to and often used as a reference in the case of trade disputes (World Health Organization, 2005). Codex assessment guidelines are based on scientific data such as chemicals, toxicological, and nutritional evaluations of the GM products and their conventional counterparts (Codex Alimentarius, 2009); thus, if GM food can be demonstrated as “substantially equivalent” to existing food or food counterpart, it can be regarded as being as safe. The Cartagena Protocol on Biosafety, on the other hand, uses the “precautionary approach” which institutes the right of a country to take into account socioeconomic considerations arising from the impact of GM products on the conservation and sustainable use of biodiversity, especially with regard to the value of biodiversity to indigenous and local communities, and the lack of scientific evidences shall not prevent a country from taking a decision, as appropriate, with regard to the import of GM products in order to avoid or minimize such potential adverse effects (Zarrilli, 2005).
WTO agreements commonly take Codex Alimentarius as a reference while the Cartagena Protocol put emphasis on local conservation and sustainable use of biodiversity, thus, countries standing on different ground of GM agreement may encounter a trade dispute on GM products. Major producers and traders of GM commodities such as Argentina, Australia, Canada, and the US do not ratify Cartagena Protocol while GM opposing countries such as the EU, Japan, South Korea, the UK and most developing countries do (Convention on Biological Diversity, 2018). Before Thailand became a member of Cartagena Protocol on Biosafety in 2006, in 2000 there was a GM trade dispute for import restriction of canned tuna fish in soybean oil from Thailand to Egypt filed for WTO consultation. More recently, according to the European Union Rapid Alert System for Food and Feed (RASFF), more than 40 shipments of papayas originating from Thailand were detected positive for GM contamination and rejected from 2013-2017 (USDA Foreign Agricultural Service, 2018). Since 2014, the DOA regulated that all exports of fresh or dried papaya or food products containing papaya to the EU and Japan are subject to GM detection testing prior to shipping. Furthermore, in 2016, formal criteria must be met for exporters of Thai fresh papaya in order to export to the EU, Switzerland, Norway, Iceland, China, and Japan (USDA Foreign Agricultural Service, 2018).
GM FOOD LABELING
GM food labeling regulations varied significantly across countries (Gruere and Rao, 2007). Codex Alimentarius Commission considers that different countries may make their own decisions on whether or not to label GM foods, and emphasizes that labelling arrangements should be in conformity with the provisions promulgated by the Codex to avoid potential trade issues (Centre for Food Safety, 2019). Policies on GM food labelling vary significantly in different countries. While some countries such as Australia, China, the E.U., New Zealand, Japan, South Korea, Switzerland, Taiwan and Thailand adopted mandatory labeling of GM food, GM food labeling in others countries such as Canada, Hong Kong and the U.S. is voluntary. The voluntary labeling approach generally requires that only GM food that is significantly different from its conventional counterpart needs to be labelled. The mandatory labeling approach can be either “pan-labeling” or “labeling for designated products only”. The “pan-labeling” requires that any food products, which contain GM materials exceeding a threshold level or are significantly different as a result of genetic modification, must be labelled. The “labelling for designated products only” requires that only the designated GM products need to be labelled (Centre for Food Safety, 2019). Furthermore, the threshold of contamination, whether it is enforced on process or product, the product coverage and exemption also varied across countries.
In December 2018, the U.S., one of the major producers of GM crops, announced the first regulation for GM food labeling which will be in effect on 1 January 2022. Under National Bioengineered Food Disclosure Standard, certain products made from the 13 GM crops and foods on the USDA’s list including papaya and pineapple, however, do not require labeling. Refined foods derived from GM crops that do not contain detectable modified genetic material are not required for GM labeling (USDA Agricultural Marketing Service, 2018).
Because producers and sellers of GM foods have more information about sources of product materials more than consumers, the market of GM foods is asymmetric information without mandatory labeling. In Thailand, the Ministry of Public Health’s Notification on GM Food Labeling 2002 adopted mandatory labeling for designated products only. It requires that 22 food items containing ingredients derived from GM soybean (and its products) and maize (and its products) more than 5% of the total weight in the top three components by weight and containing more than 5% of GM components of each ingredient are subject to mandatory GM food labeling (Ministry of Public Health, 2002). The list of 22 products subject to mandatory positive labeling includes 1) soybeans, 2) cooked soybeans, 3) roasted soybeans, 4) bottled or canned soybeans or soybeans contained in retort pouch, 5) natto, 6) miso, 7) tofu or tofu fried in oil, 8) frozen tofu, 9) soybean gluten from tofu or its products, 9) soybean milk, 10) soybean flour, 11) food containing product(s) from 1 to 10 as the main ingredient, 12) food containing soybean protein as main ingredient, 13) food containing green soybean as main ingredient, 14) food containing soybean sprout as main ingredient, 15) corn, 16) popcorn, 17) frozen or chilled corn, 18) bottled or canned corn or corn contained in heat-treated pouch, 19) corn flour or cornstarch, 20) snack foods deriving from corn as main ingredient, 21) food containing product(s) from 15 to 20 as the main ingredient, and 22) food containing corn grits as main ingredient.
Positive GM labeling enforces that food containing only one main ingredient should include a statement of “genetically modified” in conjunction with, or in close proximity to, the name of the food such as “genetically modified corn” or “tofu produced from genetically modified soybean”. For multi-ingredient products, labels should include a statement of “genetically modified” in conjunction with, or in close proximity to, or under the names of top three main ingredients of the food product such as “genetically modified corn starch”. Unlike voluntary negative labeling that provides information about the absence of GM ingredients, the use of “GMO-free”, “non-GM”, “does not contain GM”, or other statements alike is prohibited in Thailand (Figure 2). The regulation, however, is not applied to small venders who produce and directly sell to consumers. Although Thailand does not allow negative labeling of GM food products, some products in the market were found to be labelled GM negatively (Figure 3). Krualee and Napasintuwong (2012) found that negative labeling may be more appropriate than positive labeling for designated products only for Thailand when the majority of consumers are averse to GM food and willing to pay less for GM contaminated products or if they consider negative health impacts a serious problem. The advantage of positive labeling for designated food products allows consumers to have information whether the designated food items contain GM materials and hence make informed choices, but it imposes significant costs of segregation, testing, certification and labeling to the food industry that may either be absorbed by the producers or passed on to the consumers Krualee and Napasintuwong (2012). Thus, should be weighed against the benefits to consumers.
Thai Food and Drug Administration is currently considering adopting a mandatory food safety dossier submission for food ingredients derived from GM crops. If this regulation is adopted, a positive list of approved GM events will be created and would be allowed to be used as food ingredients. The final rule is projected to be approved in late 2019 (USDA Foreign Agricultural Service, 2018).
CONCLUSION
As modern biotechnology has shown positive impacts in several countries, the adoption of GM crops has increased after its first commercialization in 1996. Countries in Southeast Asia including Myanmar, Philippines, and Vietnam have legalized GM maize production. Although Thailand started the field trials of GM crops as early as in the 1900s, but due to the lack of Biosafety legislation and a precautionary delay, presently there is no open field trials nor commercial production of GM crops. Undecided policies and regulations have prohibited the progress of the recognized potential benefits from transgenic technology even though the use of modern biotechnology such as Marker Assisted Selection and genomic and bioinformatics and others are being supported by the government. While restrictions on production and imports of GM crops continue, Thailand does allow importation of GM soybeans, GM maize, GM cotton lints, and GM processed food. At the same time, Vietnam and Myanmar have approved GM crop cultivation. As in the case of GM papaya contamination in papaya products from Thailand, it is likely that GM seeds will cross the border from neighboring countries and create difficulties to regulate unauthorized GM crops in the future unless Biosafety legislation is enacted. This paper has provided situations on R&D, regulations and status on GM crop and food products in Thailand. It is suggested that Thailand will have to consider changes for appropriate policies in response to changes in global biotechnology advancement.
REFERENCES
Biosafety Clearing House of Thailand. 2009. Status of research and development of genetically modified plants. Office of Natural Resources and Environmental Policy and Planning. ( http://bch-thai.onep.go.th/status_research_develop_plant.html; Accessed 20 May 2019)
Centre for Food Safety. 2019. International development in labelling of GM foods
(https://www.cfs.gov.hk/english/programme/programme_gmf/programme_gmf_gi_... Accessed 20 May 2019)
Convention on Biological Diversity. 2018. Parties to the Cartagena Protocol and its supplementary protocol on liability and redress. (https://bch.cbd.int/protocol/parties/; Accessed 20 May 2019)
Center for Agricultural Biotechnology. 2010. About the center.
(http://www.cab.ku.ac.th/CAB-2010-04/aboutme.php; Accessed 20 May 2019)
Codex Alimentarius Commission. 2009. Food derived from modern biotechnology. Second edition.
(http://www.fao.org/3/a1554e/a1554e00.htm; Accessed 20 May 2019)
Esparanza, V.L., Alpuerto, B., Norton, G.W., Alwang, J., and Ismail, M. 2009. Economic impact analysis of marker-assisted breeding for tolerance to salinity and phosphorous deficiency in rice. Review of Agricultural Economics 31(4): 779–92.
Eşa, I., Gavahianb, M., Marti-Quijalc, F.J., Lorenzod, J.M., Khaneghahe, A.M., Tsatsanisf, C., Kampranisg, S.C., and Barbac, F.J. 2019. Biotechnology Advances 37(3): 410-421.
Gruere, G. P., and Rao, S. R. 2007. A review of international labeling policies of genetically modified food to evaluate India’s proposed rule. AgBioForum 10(1): 51-64.
Krualee, S., and Napasintuwong, O. 2012. Consumers’ willingness to pay for non-GM food labeling in Thailand. International Food Research Journal 19(4): 1375-1382.
Meesincee, S. (2018). Adaptation of agriculture and food industry to Thailand 4.0. Dinner Talk organized by the Agricultural Economics Society of Thailand under Royal Patronage. 27 September 2018. Centara Grand at Central Ladprao, Bangkok.
Ministry of Public Health. 2002. Notification on labeling regulation of transgenic food or food derived from genetic engineering technique (No. 251) B.E. 2545 (2002). (in Thai)
Napasintuwong, O. (2011). The rice of rice biotechnology. Farm Policy Journal 8(1): 55-65.
Napasintuwong, O. (2010). The role of agricultural biotechnology policies in Thailand’s economy. Asian Biotechnology and Development Review 12(1): 1-19.
Napasintuwong, O., and Traxler, G. J. 2009. Ex-ante impact assessment of GM-papaya adoption in Thailand. AgBioForum 12(2): 209-217.
Napasintuwong, O. and Traxler, G.J. 2007. Ex-ante economic evaluation of transgenic plants adoption in Thailand. Kasetsart University Journal of Economics 14(2): 86-102. (in Thai).
Narkprasert, D., and Thammakijjawat, P. 2018. Country status report—Thailand. Regional Expert Consultation on Agricultural Biotechnology – Scoping Partnerships to Improve Livelihoods of
Farmers in Asia and the Pacific. Strategic Papers and Country Status Reports. Bangkok, Thailand; 29-31 May 2018.
National Center for Genetic Engineering and Biotechnology (BIOTEC). 2018. Research Unit.
(http://www.biotec.or.th/en/index.php/research/research-units; Accessed 20 May 2019)
National Center for Genetic Engineering and Biotechnology (BIOTEC). 2010. White paper: Updated Status and Perspective of Thailand on Research and Development of Modern Biotechnology and Biosafety Regulation. 3rd Edition. Technical Biosafety Committee, Thailand.
National Science Technology and Innovation Policy Office (STI). 2011. Thailand’s National Biotechnology Policy Framework (2012-2021). Ministry of Science and Technology, Thailand
Office of the National Economic and Social Development Board (NESDB). 2017. The 12th National Economic and Social Development Plan, 2017–2021. (http://www.nesdb.go.th; Accessed 20 May 2019) (in Thai)
Pandey, S., and Rajatasereekul, S. 1999. Economics of plant breeding: the value of shorter breeding cycles for rice in Northeast Thailand, Field Crops Research 64(1–2): 187–97.
Royal Thai Embassy, Washington D.C. (2015) Biotechnology. Industry in Thailand
(https://thaiembdc.org/thai-industries/; Accessed 20 May 2019)
Shew, A. M., Nalley, L. L., Snell, H. A., Nayga, R. M., Jr., and Dixon, B. L. 2018. CRISPR versus GMOs: Public acceptance and valuation. Global Food Security 19: 71-80.
Thammasat University Economic Academic Service Center. 2014. Study on the economic and social impacts of genetically modified crops in Thailand. Report submitted to National Science Technology and Innovation Policy Office. (in Thai)
Thailand Board of Investment. 2017. Thailand’s Bioeconomy Industry.
(https://www.boi.go.th/index.php?page=brochure; Accessed 20 May 2019)
UNESCO. 2019. Data for the sustainable development goals. Thailand—Science, technology and innovation indicators. Expenditure on R&D
(http://uis.unesco.org/en/country/th?theme=science-technology-and-innovat... Accessed 20 May 2019)
United Nations Conference on Trade and Development (UNCTD). 2015. Science, technology and innovation policy review – Thailand. United Nations, New York and Geneva.
USDA Agricultural Marketing Service. 2018. Bioengineered food disclosure and labeling.
(https://www.ams.usda.gov/rules-regulations/be; Accessed 20 May 2019)
USDA Foreign Agricultural Service. 2018. Thailand Agricultural Biotechnology Annual 2018. Global Agricultural Information Network (GAIN) Report Number: TH8166. 20 December 2018. (https://www.fas.usda.gov/data/thailand-agricultural-biotechnology-annual-1; Accessed 20 May 2019)
World Health Organization. 2014. Frequently asked questions on genetically modified foods.
(https://www.who.int/foodsafety/areas_work/food-technology/faq-geneticall... Accessed 20 May 2019)
World Trade Organization. 2005. Modern food biotechnology, human health and development: An evidence-based study. (https://apps.who.int/iris/handle/10665/43195; Accessed 20 May 2019)
Zarrilli, S. 2005. International trade in GMOs and GM products: National and multinational legal frameworks. United Nations Conference on Trade and Development. Policy Issues in International Trade and Commodities, Study Series No. 29.
Date submitted: May. 30, 2019
Reviewed, edited and uploaded: June. 13, 2019